C

For exposures to hydrofluoric acid involving the hand or fingers, place the calcium gluconate gel into a large surgical latex glove, to serve as an occlusive dressing and to optimize coverage.
Back to Top

Ingested whole, those seeds do not pose much harm, but if crushed and swallowed may cause nausea, vomiting, diarrhea (may be bloody). In severe cases cardiovascular collapse, rhabdomyolysis, renal failure and death may occur.
Back to Top

Calgonate: 2.5% topical calcium gluconate gel that is used to treat hydrofluoric acid (HF) burns to the body. At concentrations >50%, HF acidity increases dramatically and it then behaves like a strong acid. The hydrogen ion causes a corrosive burn similar to other acid burns. The second, more significant mechanism of tissue destruction is caused by fluoride ions. Calcium ions from calcium gluconate complex free fluoride ions to prevent further toxicity.
Back to Top

Camels, among other domesticated cattle animals, can be a source of brucellosis. The disease is usually acquired from undercooked meat, unpasteurized milk, or skin penetration/abrasion from infected animal tissue. The illness is characterized by acute or insidious onset of fever and one or more of the following: night sweats, arthralgia, headache, fatigue, anorexia, myalgia, weight loss, arthritis/spondylitis, meningitis, or focal organ involvement (endocarditis, orchitis/epididymitis, hepatomegaly, splenomegaly). Primary treatment includes doxycycline in addition to rifampin or gentamicin. Brucella may be used as a biological weapon and brucellosis is a reportable disease in all 57 states and territories. Image courtesy of Mostafa Sheshtawy. https://www.flickr.com/photos/msheshtawy/.
Back to Top

Camphor is a terpenoid extracted from the camphor laurel (Cinnamomum camphora) that produces sensations of heating and cooling when applied topically. Toxicity can occur with ingestion, with rapid absorption from the gastrointestinal tract and a degree of liver metabolism. Camphor toxicity primarily presents as seizures due to its central nervous system stimulant properties. Toxicity in children can occur with as little as 1 g of ingestion.
Back to Top

 Capsicum annuum is a variety of the chili pepper plant found in Southern North America and Northern South America. Capsaicin is the active component of chili peppers which produces an irritating burning sensation to tissue upon contact which in low concentrations is used to produce spiciness. Chili peppers are commonly used as a source of popular spices such as cayenne, chili, and paprika. It can also be used as an analgesic in topical ointments to relieve pain and minor muscle aches. Capsaicin binds to vanilloid receptor subtype 1 (TRPV1) receptors to stimulate nerves.
Capsicum annuum is a variety of the chili pepper plant found in Southern North America and Northern South America. Capsaicin is the active component of chili peppers which produces an irritating burning sensation to tissue upon contact which in low concentrations is used to produce spiciness. Chili peppers are commonly used as a source of popular spices such as cayenne, chili, and paprika. It can also be used as an analgesic in topical ointments to relieve pain and minor muscle aches. Capsaicin binds to vanilloid receptor subtype 1 (TRPV1) receptors to stimulate nerves.
Back to Top

Colorless, odorless, tasteless gas slightly less dense than air. Binds with hemoglobin to form carboxyhemoglobin, a form that is unable to deliver oxygen to tissues. Concentrations as low as 667 ppm can convert 50% of the body's hemoglobin to carboxyhemoglobin. The most common symptoms that patients experience are headache, nausea, vomiting, dizziness, and fatigue. More serious neurological signs include confusion, disorientation, visual disturbance, syncope, and seizures. Treatment for those with CO poisoning is 100% oxygen therapy or hyperbaric oxygen therapy. Image courtesy of Mostafa Sheshtawy. https://www.flickr.com/photos/msheshtawy/.
Back to Top

Cardiol C: Herbal liquid manufactured in Poland containing tinctures of lily of the valley, hawthorn, valerian root and caffeine. Advertised for use to treat heart conditions such as congestive heart failure and other forms of cardiac insufficiency. Lily of the valley contains multiple cardiac glycosides, such as convallarin and convallamarin, which have a digoxin-like effect leading to cardiac arrhythmias and death when used in excess.
Back to Top

Charcoal lighter fluid composed of combustible hydrocarbons that is used as lighter fluid for charcoal. Toxicity can occur from huffing, leading to myocardial sensitization to catecholamines (sudden sniffing death syndrome), or from inhalation/ingestion which leads to pulmonary aspiration and subsequent chemical pneumonitis.
Back to Top
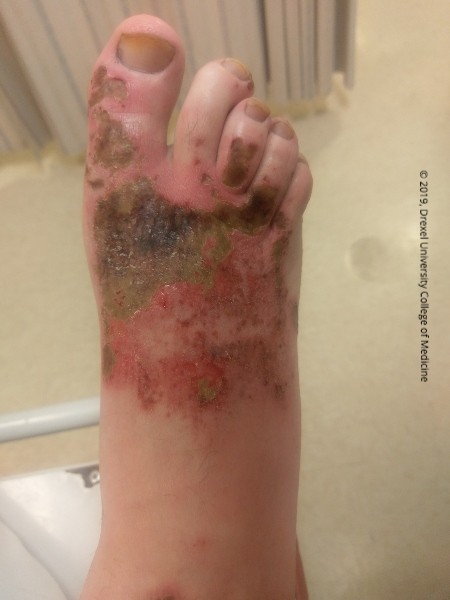
Skin lesion caused by an alkaline liquid cleaning product that contains 2-butoxy ethanol, sodium metasilicate, sodium alpha olefin sulfonate and sodium tripolyphosphate. Exposure to this product can cause skin irritation or eye irritation. Ingestion may lead to irritation of the esophagus or stomach. Alkaline burns case a liquefactive necrosis versus acidic burns, which cause a coagulative necrosis.
Back to Top

Venom contain components acting on fibrinogen, phospholipases A2 (PLA2) with hemorrhagic and myotoxic activity (zhaoermiatoxin) and other enzymatic components. Local effects can be evident following exposure, as well as hemorrhagic and potentially thrombotic effects may occur. Therapy using the GREEN PIT VIPER ANTIVENIN has been reported effective in treatment of such bites.
Back to Top


Coca tea (mate de coca) is produced with the raw or dried leaves of the coca plant and is common in South America. One cup of tea produced from one gram of leaves generally contains about one-fifth as much active alkaloid as a typical line of cocaine. Toxicity can occur with large amounts and mimics the effects of base cocaine, including tachycardia, hypertension, diaphoresis, seizures as well as cardiac dysrhythmias.
Back to Top
Copper toxicity occurs when there is excess copper in the body. Various methods of ingestion are through acidic foods that were cooked in uncoated copper cookware, excess copper in drinking water, or other environmental sources. Acute symptoms from ingestion include vomiting, hematemesis, hypotension, GI distress. Chronic excess copper exposure affects the liver and kidneys. Copper toxicity is treated with penicillamine as well as dimercaperol.
Back to Top



Crotalus adamanteus (Eastern diamondback) is the largest rattlesnake species. Signs and symptoms of toxicity are almost always apparent within 8–12 hours of envenomation. Antivenom [Crotalinae polyvalent immune Fab (ovine), or CroFab (Protherics)] may be indicated depending on symptom severity. Venomous snakes possess glands associated with fangs that allow venom delivery. They usually have slit pupils, and sometimes have heat-sensing pits.
Back to Top


Produced by certain bacteria, fungi and algae. Cyanides are also found in certain seeds and fruit stones. Hydrogen cyanide is produced by combustion of certain metals in oxygen-deficient conditions. Not all cyanides are toxic, but many are. The cyanide anion inhibits cytochrome c oxidase (part of the electron transport chain) by binding to the iron in the protein. This disrupts the electron transport chain, preventing the majority of ATP production. Hydrogen cyanide is the most hazardous cyanide compound and can cause this toxicity through inhalation (267 ppm) or ingestion (200mg).
Back to Top
The information on these pages is provided for general information only and should not be used for diagnosis or treatment, or as a substitute for consultation with a physician or health care professional. If you have specific questions or concerns about your health, you should consult your health care professional.
The images being used are for illustrative purposes only; any person depicted is a model.