Research Projects
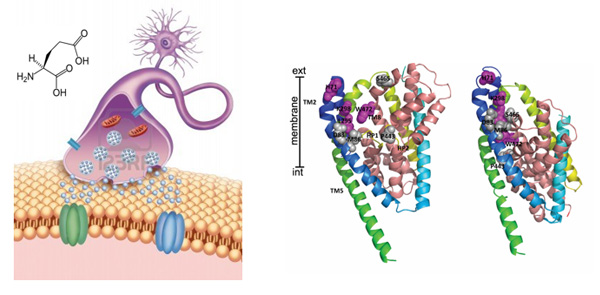
The glial glutamate transporter EAAT2 plays a major role in glutamate clearance in synaptic clefts. Removal of excess cellular glutamate has been strongly implicated as a clinically relevant means to treat neurodegenerative diseases where excitotoxicity contributes to neuronal injury and death. In this regard, EAAT2 is considered a promising drug target for the treatment of numerous conditions that involve glutamate excitotoxicity.
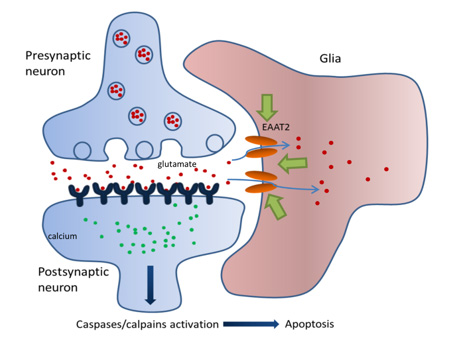
Although significant effort has been devoted toward the identification of EAAT2 activators, there has been limited success thus far in identifying direct and potent EAAT2 activators with neuroprotective properties.
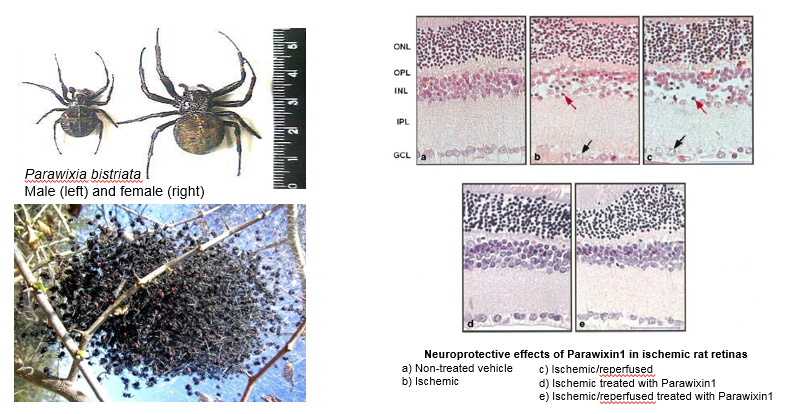
Neurotoxins from animal sources are powerful tools in neuroscience. Our lab's interest in glutamate transporters started when we discovered a unique natural compound purified from the venom of the spider Parawixia bistriata, referred to as Parawixin1. This compound was shown to enhance glutamate transport by acting specifically on EAAT2 and to have a neuroprotective effect on retinal tissue from ischemic damage.
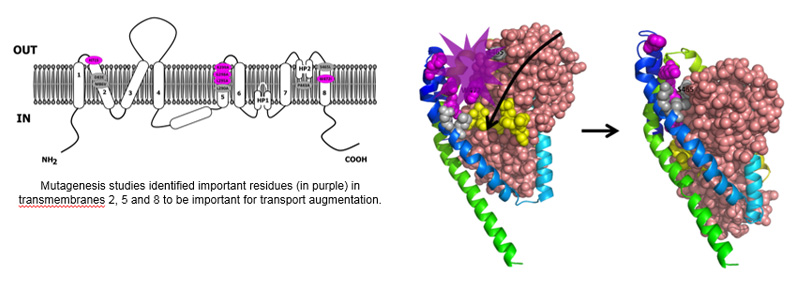
Using mutagenesis studies, we have identified molecular determinants involved in EAAT2 transport enhancement by Parawixin1.
![Novel EAAT2 PAM [M]](/~/media/Images/medicine/departments/department-pharma-physiology/Drexel_Mortensen_Novel_EAAT2_PAM_M.ashx?la=en)
Using this distinctive information, a hybrid structure-based virtual screening was applied and has identified novel EAAT2 activating compounds. These compounds have been characterized to be small positive molecules allosteric modulators (PAM) of EAAT2.
We believe that EAAT2 PAMs are advantageous over other clinical approaches targeting processes involved in excitotoxic events. For instance, glutamate receptor blockers have been shown to alleviate cellular damage and neurologic deficits after brain trauma; however, serious side effects limit their translation to the clinic. Besides, approaches that rely on increasing levels of EAAT2 protein expression, such as ß-lactam antibiotics, have been shown to be neuroprotective, but this approach is best administered prophylactically, having low relevance for acute conditions. Also, this approach could be associated with non-specific effects that limit their utility.
Our lab is also interested in traumatic brain injury (TBI). Worldwide, TBI is a major cause of injury-induced mortality and morbidity with very few options to limit acute and long-term progression of secondary injury. Human and animal studies on TBI demonstrated an acute increase in tissue glutamate concentrations that remain high for up to five days in humans, suggesting a lack of timely clearance by glutamate transporters.
We have analyzed the effects of the compound MS-153 ([R]-[-]-5-methyl-1-nicotinoyl-2-pyrazoline), a synthetic EAAT2 activator, on the pathology of TBI. First, we have established that the activity of EAAT2/GLT-1 is significantly decreased after lateral fluid percussion (a rodent model of TBI) in the injured hemisphere of rats. Then, we determined the compound to be effective in preventing neurodegeneration, MAP-2 loss and calpain activation after TBI.
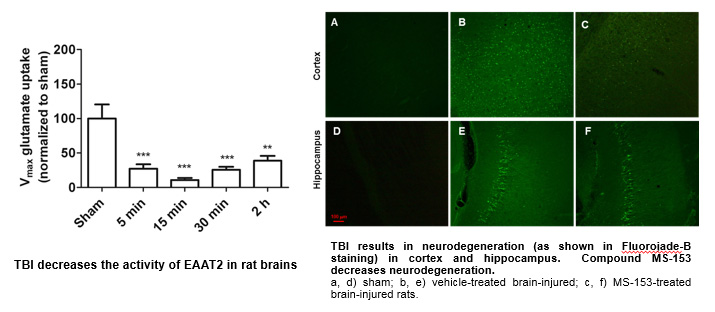
These intriguing and exciting data with MS-153 emboldened us to take this approach to the next level and examine the effects of our PAMs on the pathology of TBI (ongoing studies).
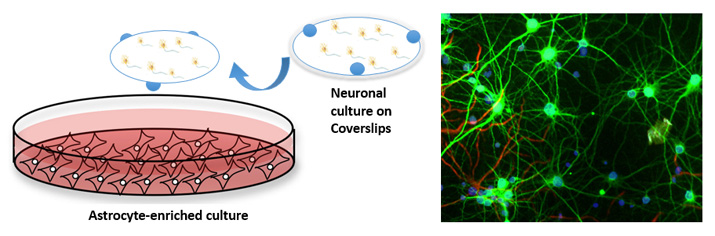
Currently, our lab is characterizing these novel PAMs of EAAT2 in in vitro models of excitotoxicity. Specifically, we aim to examine whether augmentation of astrocytic glutamate reuptake provides neuroprotection after glutamate excitotoxicity and in vitro TBI. We are employing models of primary cortical neuron cultures subjected to excitotoxic insults (such as glutamate, NMDA, transient deprivation of oxygen and glucose and oxidative stress). We are also investigating primary hippocampal neurons subjected to stretch injury, an in vitro model of TBI.
Additionally, medicinal chemistry studies aim to further characterize the drug-like properties of these compounds, including the determination of the best analogs of our PAMs for pre-clinical studies, determination of pharmacokinetic and pharmacodynamics parameters, further optimization of dose and delivery methods of the compounds, and comprehensive safety testing. Regarding TBI studies, we also aim to determine the therapeutic window of efficacy for treatment on different models and severities of brain injury.
Our lab is also interested in epilepsy since glutamate plays a role in the initiation and spread of seizure activity and also plays a critical role in epileptogenesis. In rodent models, altered glutamate receptor or glutamate transporter expression by knockout or knockdown procedures can induce or suppress epileptic seizures. Also, microdialysis studies show an increase in the extracellular concentration of glutamate and aspartate before or during seizure onset. In this regard, PAMs of EAAT2 could be developed as a therapy for prevention and/or suppression of epileptic seizures. Also, there is evidence that increased EAAT2 expression reduces epileptogenic processes following pilocarpine-induced status epilepticus. Our lab is initiating a collaborative project aiming to examine the intriguing possibility that PAMs of EAAT2 could be neuroprotective. Specifically, we will examine an in vitro model of acquired epilepsy in hippocampal neurons and in vivo pilocarpine and ketamine models of epilepsy.
Our long-term goal is to progress our pre-clinical studies with this class of PAMs of EAAT2 by evaluating translational possibilities in in vivo models of neurological disorders such as brain injury and epilepsy, and we hope to begin clinical trials in patients of relevant neurological disorders.
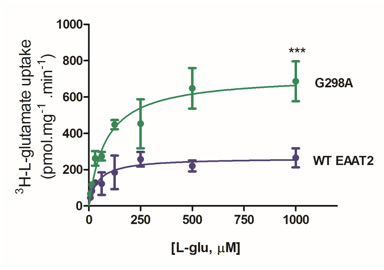
Furthermore, our lab is currently examining structural components of EAAT2 that, when mutated, result in increased EAAT2 activity. Intriguingly, mutation of the glycine residue at position 298 to alanine resulted in augmented rate of glutamate transport levels. Structure-function relationship, cell surface protein expression, and computational modeling studies have suggested that this mutation of glycine to a greasier alanine resulted in facilitated transport. Ongoing high-impact dynamic studies will provide insights into the conformations and stabilization of EAAT2 in this mutation and additional ones.
This work has implications for understanding mechanisms for glutamate transport up regulation and, as a result, how to target EAAT2 for neuroprotective strategies in the future.
Current projects in the lab involve the examination of the effects of these compounds in in vitro and in vivo models, that include, among others, stroke, TBI, epilepsy, neuropathic pain, and cocaine and alcohol use disorders.
One current project also involves a recently awarded grant from NINDS to understand the molecular mechanisms of allosteric modulation of glutamate transporters. To reach this goal, we will obtain and integrate multidisciplinary knowledge of the transport function, 3D structures, and single-molecule dynamics of the transporters and their complexes with the allosteric compounds. In turn, this knowledge will aid understanding the basic membrane transport mechanisms of the most important excitatory neurotransmitter the human brain.
Back to Top