Research
Spinal circuits for sensorimotor integration and interlimb coordination during locomotion
NIH/NINDS R01 NS115900; 09/21/2020 - 06/30/2025
PI: Simon M. Danner; Co-PI: Turgay Akay (Dalhousie University)
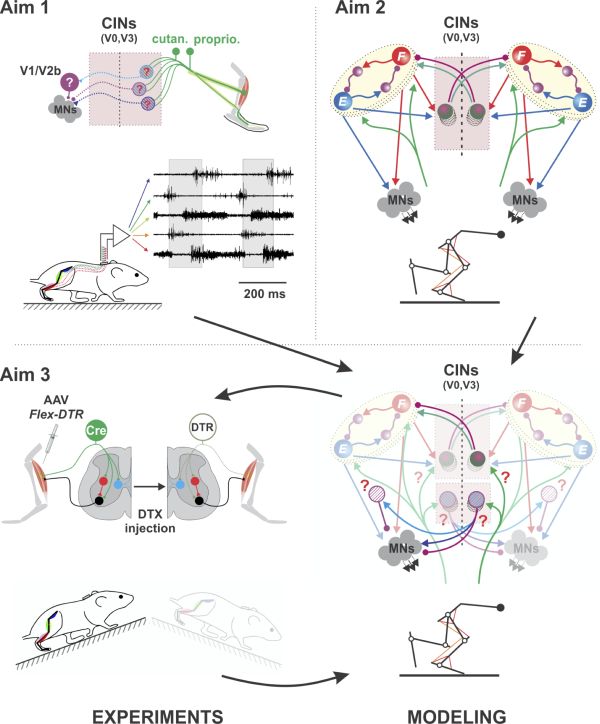
Somatosensory feedback from the limbs is essential for locomotion and its recovery after spinal cord injury. To achieve stable locomotion, the spinal cord needs to process afferent feedback signals and properly adjust muscle activation and interlimb coordination. Crossed-reflex pathways, specifically, are important for gait stability and balance, which are impaired in various motor disorders and in the elderly. Recently, significant progress has been made in decoding the organization and function of the central spinal locomotor circuitry and its brainstem command system. But the interactions of somatosensory feedback with the spinal circuitry during locomotion have yet to be understood on the same level of detail.
In this project we propose to address this gap of knowledge by combing mouse genetics, in vivo electrophysiology and behavioral analyses with computational modeling of spinal circuits and the musculoskeletal system to systematically dissect sensory afferent connectivity to the locomotor circuitry, including genetically identified neuron populations, and their function in interlimb coordination. Studying the organization of crossed reflexes and their interactions with spinal locomotor circuitry will provide critical information for rehabilitative strategies.
This multidisciplinary project will be performed in close interactive collaboration between two investigators with strong and complementary expertise in computational (Simon Danner, PI) and experimental studies of neural control of locomotion (Turgay Akay, co-PI). The project has the following three aims:
- Delineate the involvement of multiple spinal interneurons in the processing of sensory information and interlimb coordination by studying crossed reflexes at rest and during locomotion.
- Design a predictive computational model of the spinal locomotor circuitry and its interactions with the mouse musculoskeletal system.
- Integrate modeling and experimentation to uncover underlying neural mechanisms.
The model will be used to derive informative predictions that will then be tested experimentally. This process has the advantage of providing an explicit and consistent theoretical framework for experimentation, thereby reducing the number of necessary experiments while increasing the information gained per experiment. In summary, the proposed multidisciplinary approach is based on state-of-the-art experimental and modeling methods and will provide important and novel insights into the neural organization of the spinal locomotor circuitry responsible for sensorimotor integration and interlimb coordination during locomotion that cannot be obtained by experimentation or modeling alone.
This project is relevant for public health because understanding the role and function of the spinal circuits controlling sensorimotor integration and interlimb coordination in the mammalian spinal cord is a prerequisite for the systematic study of impaired locomotion due to a variety of syndromes. Additionally, it will establish a theoretical foundation for clinical research aiming to improve locomotor function following spinal cord injury and degenerative disorders.
Back to Top
Propriospinal neuron function in normal and post-SCI locomotion
NIH/NINDS R01 NS112304; 09/2020–08/2025
PIs: David S. K. Magnuson (University of Louisville), Simon M. Danner, Scott R. Whittemore (University of Louisville)
Despite the more than 100 years since the recognition of intrinsic spinal locomotor circuits, many of the functional details of those circuits and their contributions to recovery following spinal cord injury (SCI) remain to be determined. Recent development of powerful molecular tools enables functional dissection of neural circuitry via reversibly silencing neurotransmission and trans-synaptic labeling. We will combine these tools with sophisticated gait and kinematic analyses, that includes the full repertoire of speed dependent gaits, to provide the functional and anatomical information necessary for building and refining an advanced neuro- biomechanical computer model of the rat spinal cord, body and limbs. We will focus on two classes of spinal cord interneurons, the long ascending (LAPNs) and descending (LDPNs) propriospinal neurons, that interconnect the forelimb and hindlimb circuits and central pattern generators in the two enlargements, and investigate their role in the intact spinal cord and after SCI using both hemisection and contusion models. Our preliminary data show that these LAPNs/LDPNs are essential components involved in speed-dependent gait expression. Silencing these neurons partially decouples the right and left limbs at each girdle. Surprisingly, silencing these neurons after an incomplete contusion injury results in better overground locomotion, a result that is hard to reconcile based on current knowledge and observations in uninjured animals. Using viral-based trans-synaptic labeling we will determine the sensory, descending and propriospinal inputs onto both LAPNs and LDPNs. We will utilize both existing and new physiological and biomechanical data (Aim 1) as well as new anatomical data (Aim 2) to build and refine our computational model (Aim 3). Then, in vivo experiments and computer modeling will be performed in parallel (Aim 4) to determine the roles that ipsilateral and commissural LAPNs and LDPNs play in locomotor behavior, including the full range of locomotor gaits, and in recovered function after hemisection and incomplete contusion injuries. We suggest that a deeper understanding of long propriospinal neurons represents an important step towards the development of new therapeutic tools for recovery after SCI.
This multi-PI project focuses on the role of the long ascending and descending propriospinal neurons (LAPNs, LDPNs) in the control of locomotion and recovery after a spinal cord injury. We will use a combination of viral based synaptic silencing, retrograde and trans-synaptic tracing and comprehensive gait and kinematic analysis to aid the development of a neuro-biomechanical computer model and to generate important new knowledge of spinal locomotor circuitry.
Back to Top