Gut Instincts in Obesity Research

The obesity epidemic is a notoriously complex problem that has confounded researchers for decades, but Nicholas V. DiPatrizio, PhD '08, has uncovered a promising new connection. His findings suggest that the body's lipid messengers in the gut, known as the endocannabinoids, signal the brain to seek out fat-rich foods — and that too much of this signaling results in the addiction-like behavior of compulsive overeating.
More than two-thirds of adults in the United States are overweight or obese, and associated diseases and conditions cost the health care system between $147 billion and $210 billion per year. "We know this is a significant problem, but we also know it's not about one system of the body," says DiPatrizio, assistant professor of biomedical sciences at the University of California Riverside School of Medicine.

Nicholas V. DiPatrizio, PhD '08
Research has shown that humans and other mammals adaptively gravitated toward fat-rich foods for survival in the wild. Yet in our contemporary world with its industrialized food system, high-caloric foods require little energy expenditure to obtain. The continued instinctive drive to eat them is a major factor in the obesity epidemic.
DiPatrizio's work focuses on the endocannabinoids, a group of lipid signaling molecules in the body that moderate numerous physiological processes. The endocannabinoids are naturally released chemicals that activate cannabinoid receptors throughout the body, which, as their name suggests, are also activated by marijuana. It is at the site of this system that a link between overeating and drug abuse may be made.
As neuroscientists, we tend to be very brain-centric but there is a body attached to the brain, and I wanted to understand how it regulates feeding and sensory signaling.
Understanding Food as a Drug
"The brain regions that are activated by overeating are those similar to the regions activated by drugs of abuse," DiPatrizio says. "So it's likely that the drugs actually highjack a natural reward system in the body that was a survival mechanism for eating."
DiPatrizio first became interested in the endocannabinoid system as a psychology major at Temple University, when he worked in a pharmacology lab to study the body's interactions with cannabis. Later, as a graduate student at Drexel College of Medicine, he approached Kenny Simansky, PhD, now vice dean for research, who was conducting studies about serotonin, opioids and food reward. Seeing a logical connection, DiPatrizio pitched him the idea of looking at endocannabinoids in the brainstem and their impact on eating patterns.
"As we train doctoral students we believe it's important to let them drive the research. When he came to me with this idea that perfectly fit into the overall purpose of my lab, I just thought it was terrific," Simansky says.
DiPatrizio regards that opportunity as the true beginning of his career. "I credit Dr. Simansky with really challenging me to think critically and rigorously. Without him, I would not have my own laboratory today."
During their collaboration, they observed rodents' eating patterns, and discovered that the endocannabinoid system in the brainstem in fact regulates neurotransmission from the oral cavity. That finding, and coursework in pharmacology and molecular biology at the College of Medicine, helped DiPatrizio think across disciplines, creating an important foundation for his future study.
If you can remotely control the urge to overeat food from the periphery, then you can reduce the probability of obesity. It's very exciting.
Following the Signals
After he completed his PhD in neuroscience at Drexel, DiPatrizio furthered his research at the University of California Irvine School of Medicine, broadening the focus to encompass an even more integrative approach. "At that point I became interested in looking at how the endocannabinoid system integrates with the brain information from other organs in the periphery. As neuroscientists, we tend to be very brain-centric but there is a body attached to the brain, and I wanted to understand how it regulates feeding and sensory signaling."
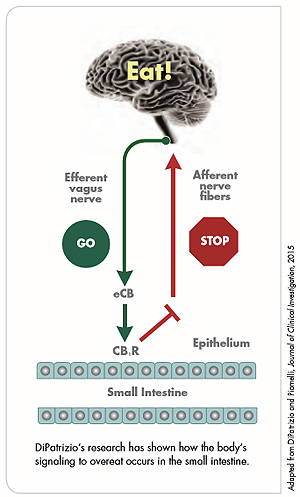
During his six-year postdoctoral fellowship, DiPatrizio began to use tandem mass spectrometry to analyze endocannabinoid levels and capture signaling events. He won NIH funding for his work, and one of his findings — that when an individual tastes dietary fats, the endocannabinoids produce signaling in an area of the small intestine — received wide media coverage, including a story in The New York Times in 2011.
Dietary Dangers
In his UC Riverside School of Medicine laboratory, DiPatrizio continues to examine the integrative neurobiology and physiology involved in food reward, sensory processing and energy balance. Twelve students in his laboratory deploy a range of approaches, including surgical, biochemical, molecular, pharmacological and behavioral tools, in addition to cutting-edge analysis with mass spectrometry and ultra-performance liquid chromatography.
As he has observed the behavioral differences of rodents eating Western (high-fat, high-sugar) food versus those on a low-fat, low-sugar diet, new questions have emerged. "As soon as you switch them to the Western diet, the rats quickly gain body weight. We look at the meal patterns and the total consumption — they end up consuming twice the number of calories as the animals eating the lean diet. It's binging behavior, much like that seen in drug addiction. We wanted to know what causes this biochemical event and encourages this way of eating."
DiPatrizio's team used mass spectrometry to look at the endocannabinoid levels in the rodents' small intestines and in their bloodstream, and found that, indeed, the levels in those rodents eating the Western diet had more than doubled.
"Our next question was whether those endocannabinoid levels were actually driving the binge-eating behaviors. Through pharmacological means, we were able to use antagonist molecules to block the cannabinoid receptors and inhibit that signaling. Sure enough, once we blocked them, we were able to normalize all feeding behaviors — meal sizes returned to normal and so did the rate of intake. Our conclusion was that the Western diet did, in fact, increase meal size associated with elevated levels of endocannabinoids." The results were shared in "Peripheral Endocannabinoid Signaling Controls Hyperphagia in Western Diet–induced Obesity," published this year in Physiology & Behavior (PMID: 28065722).
DiPatrizio now wants to analyze the diet and find out which kinds of fats, which kinds of sugars, and which combinations thereof drive the excessive signaling. "What we do know so far is that both fat and sugar are important in causing obesity," he says.
A Path to Treatment
Gaining a better understanding of the neural and molecular mechanisms underlying food preferences and compulsive eating behaviors may be essential for a broader view of the many systems in the body involved in obesity. The goal, of course, would be to apply these discoveries to the development of novel therapeutics and expand treatment options for people struggling with weight issues and resulting metabolic syndrome. An antagonist like the one DiPatrizio's lab tested, for example, might be used by humans to turn off the signaling and reduce binge eating.
A similar tactic has been tried before, but the product never made it to the American market. "About 10 years ago there was a molecule that inhibited the endocannabinoid system, and it worked very well for reducing waist circumference," DiPatrizio says. "But it was also tied to a higher incidence of depression and other psychiatric issues, so the FDA did not approve it."
DiPatrizio's focus on the peripheral nervous system may pave the way for a more successful product. "The work we've done has now identified the area in the gut that can be targeted by an inhibitor which doesn't go through the blood-brain barrier. With the right molecule you would have the same pro-metabolic effects but without the psychiatric side effects. If you can remotely control the urge to overeat food from the periphery, then you can reduce the probability of obesity. It's very exciting."
While there's much more to be learned, DiPatrizio's novel revelations about the brain-gut dialogue mark an important contribution to the body of research. "What is profound is the way this work looks beyond the brain and how what happens in our intestines can be driving our behavior," Simansky says. "He's the only one who has put this together — he took what he did in our laboratory and combined it with other findings to create a new paradigm. It's what we hope for, that a scientist comes to us with a passion and leaves with a career of discovery."
Back to Top