Research
Project #1: The highly enriched c-type lectins on dendritic cells provide potential therapeutic strategy to ameliorate neuroinflammatory diseases
Thus far, our seminal contribution lies in bridging two important fields of neuroscience and immunology while strengthening DCs’ presence and functions within CNS. This is by means of our original work providing direct evidence for the ability of circulating DCs to migrate across the inflamed BBB during an active ongoing neuroinflammatory condition (EAE) by live intravital videomicroscopy. This was further substantiated by a variety of non-invasive imaging tools such as NIRF, SPECT-CT and PET-based in vivo imaging (Fig. 1).
The mechanism by which DCs are recruited across the BBB during neuroinflammation has been the least explored amongst all leukocytes. For cells of myeloid origin, lectins and integrins are two major groups of receptors involved in trafficking cascade. While integrins function at the level of adhesion, the importance of lectins (highly enriched on DCs) remains unknown. We have identified functions of one C-type lectin receptor (mainly CLEC12A) in facilitating DCs binding and transmigration across the BBB in response to CCL2 chemotaxis. Specific antibody blocking of CLEC12A significantly ameliorated the course of experimental autoimmune encephalomyelitis in mice through an inhibition of myeloid cell infiltration into the brain and spinal cord. These studies revealed the utility of a DC-specific mechanism in designing new therapeutics for multiple sclerosis (MS). We now wish to proceed with pre-clinical efficacy and toxicity testing followed by phase I clinical trial for CLEC12A blockade as potential new therapy for the management of MS.
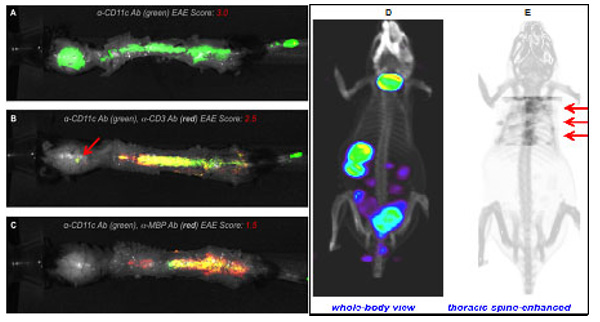
Figure 1. NIRF and SPECT-CT imaging of leukocyte presence in EAE CNS lesions. Mice were injected with either (1) anti-CD11c Ab-IRDye800, (2) anti-CD11c Ab-IRDye800+anti-CD3 Ab-IRDye680 (T cells) or (3) anti-CD11c Ab-IRDye800+anti-MBP Ab-IRDye680 on EAE day 14 and imaged 48 h post-antibody using ex vivo NIRF imaging to validate the ability to track cells to EAE lesions. A) Anti-CD11c antibody only (green) signal from DCs in a mouse with severe EAE. B) Mouse with moderate EAE score shows signal from both CD11c+ DCs (green) and CD3+ T cells (red). C) Mouse exhibiting mild EAE shows a high degree of co-localization between CD11c+ DCs and MBP signal. D) 3D rendered view of whole-body α-CD68 Ab distribution at 48h post-tracer showing mostly thyroid, stomach, spleen, and gut. E) An enhanced view of tracer uptake in thoracic spine. Red arrows denote spine uptake.
Project #2: Targeting MEF-2:HDACIIa complex as therapeutic strategy for ATLL
Lymphomas are the most common type of blood cancers, with two main forms, namely Hodgkin’s and non-Hodgkin’s (NHL), which are characterized by the abnormal growth of lymphocytes. Adult T-cell leukemia/lymphoma (ATLL) is a unique and highly aggressive form of NHL caused by the human T-cell leukemia virus type 1 (HTLV-1) with no effective treatment, cure or vaccine thus far. ATLL is a malignancy of mature CD4 T-cells with frequent visceral involvement, lymphadenopathy, hypercalcemia and monoclonal proliferation of tumor cells known as “flower cells.” These cells exhibit an unusual morphology with lobulated nuclei, and robustly express IL-2 receptor α or CD25. In recent studies, we have identified a novel role for the myocyte enhancer factor-2 (MEF-2) in HTLV-1 gene expression and associated T-cell transformation involving two key viral proteins Tax and HBZ. We are now characterizing roles of MEF-2 in the development of ATLL and test a unique strategy to curb its activity.
MEF-2 is held transcriptionally silent via histone deacetylases (HDACs) that maintain chromatin in a condensed hypoacetylated state. Because of their widespread influence on oncogenesis, HDACs have become the target of an entire class of anticancer drugs, known as histone deacetylase inhibitors (HDACi) that are constantly being explored for better isoform selectivity and fewer side effects. Interestingly, a particular class IIa inhibitor MC1568, with an unique mode of action is capable of stabilizing MEF-2:HDACIIa complex by inhibiting HDAC4 (but not HDAC3, class I) activity and enhancing their interactions, thus keeping MEF-2 tethered to the HDAC4-HDAC3 inhibitory complex and blocking its transcriptional activity in the context of myogenesis. We propose to determine if this isoform-selective HDACi can exhibit similar effects within HTLV-1-infected T-cells in vitro, in clinical samples ex vivo, and in an animal model of ATLL. The accumulation of MEF-2 within cytoplasm as a result of MC1568 may lead to the induction of autophagy and eventual death of cancer cells, which will be investigated in detail during treatment strategies.
Our goal is to systematically identify predominantly altered MEF-2 isoforms in the context of ATLL, and evaluate HDACIIa:MEF-2 stabilizing inhibitor against cancerous T-cell growth while sparing normal T cells and causing minimal off-target effects. The specific strategies to achieve these goals are to: 1) investigate MEF-2 isoforms, and target oncogene expression in a variety of HTLV-1-infected cell lines and primary cells as well as in ATLL patients; 2) test MC1568 against HDAC/MEF-2 activity, viral gene expression, T-cell growth/proliferation, and the induction of autophagy/death within cancer cells; and 3) pre-clinical testing of MC1568 in a humanized mouse model of HTLV-1 chronic infection and ATLL (IBMI-huNOG). These studies will advance the current understanding of a human chronic viral infection and bring the field closer to finding a better treatment or cure for HTLV-1-associated malignancies as well as those of other etiologies.
Project #3: Pre-clinical testing of a novel immunotherapy for HTLV-induced neurologic disease
Worldwide, 20 million people are infected with HTLV-1. A majority of them remain asymptomatic carriers (ACs), while a few develop ATLL or HAM/TSP with no effective treatment or vaccine for either disease state. The exact mechanism(s) of disease pathophysiology remain unresolved with a big question of high proviral load in HAM/TSP patients despite vigorous cellular immune response (primarily directed towards viral transactivator protein Tax).
Our initial studies implicated programmed death (PD)-1 receptor and its ligand, PD-L1, as potential underlying factors for observed immune cells’ dysfunctions leading to viral persistence and disease progression, primarily in HAM/TSP patients. PD-1:PD-L1/PD-L2 are the members of immunoglobulin superfamily (IgSF) co-signaling molecules and have been linked with CD8 T-cell exhaustion during chronic viral infections. Several members of this family (shown in Fig. 2) play a critical role in regulating antigen-specific immune responses.
Thus far, PD-1 and CTLA-4 pathways have been extensively studied; blocking antibodies against these have shown clinical benefit in the setting of both cancer and chronic viral infections. More recent data suggest that blocking multiple inhibitory receptors simultaneously may improve T-cell-based therapies, but further studies are required to clarify the role of each inhibitory receptor-ligand pair, as listed above. Moreover, the clinical applicability of checkpoint blockade remains to be tested with respect to neuroinflammatory diseases, especially those associated with chronic infection, such as HAM/TSP, neuroAIDS, etc. Interestingly, HTLV-1 provides a good model for both, and thus we find it significant to investigate the role of key inhibitory receptors/ligands in HTLV-1 infection and test their combined blockade as potential immunotherapeutic strategy to restore immune cell functions in HAM/TSP patients.
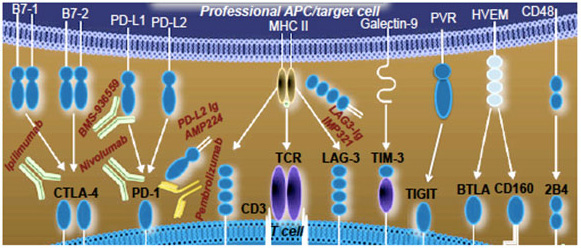
Figure 2. Inhibitory receptor:ligand pairs to be tested along with current therapeutics, both monoclonal Abs and Ig fusion proteins, approved or at various stages of clinical trials.
Back to Top