Exploring the Unknowns of Aging

Researchers are tackling the riddles with some exciting results.
By Elisa Ludwig
From metabolic processes to genomics to immunology, aging is a complex, multifaceted phenomenon that impacts the body's structure and function. While biologists have started to grasp some of the fundamental cellular changes that occur in later life, new questions about how and why we age are driving dozens of research endeavors by College of Medicine faculty and alumni.
"The basic biology of aging has evolved to the point where we understand some of the mechanisms behind how we age. We are now grappling with how to move these discoveries into clinical use," says Christian Sell, PhD, an associate professor and director of The Aging Initiative, which supports aging research (including a fellowship) at the College of Medicine. "That's a complicated task for any disease state, but it's especially complicated for aging, given factors such as timeframes and drug interactions."
The goal is not to prolong life, per se, but rather to improve quality of life, health care and potentially — in the distance — health care spending.
Better Living Through Biochemistry
Sell's area of interest is the mechanisms of lifespan extension, specifically metabolism and how cells use energy as they age. Like many researchers in the field, he has focused on the drug rapamycin, already approved by the FDA as an immunosuppressant for use in organ transplants. Rapamycin inhibits mTOR (mammalian target of rapamycin), a protein that is a core component of a network of intracellular and environmental signals that regulate cell growth and some of the biological processes that control aging.
In Sell's laboratory, rapamycin has been used in very low doses to inhibit the mTOR pathway in mice, which modulates their immune systems. The idea is that it might offer some protection from age-related disease, increasing both health span and lifespan.
"We find that in small doses, it actually improves cell function, and that has been very exciting to see," he says.
In his latest published research, Sell and his team tested the effects of a topical formulation of rapamycin in a prospective controlled study to see if it had an impact on aging in human skin.* In fact, the drug showed great promise, reducing markers of senescence and clinical signs of aging in the skin of the majority of subjects (see "How It Works").
"Used in this way, rapamycin turned out to have surprisingly good results," Sell says. "That might tell us something about how other organ systems will react and how other diseases can be addressed with this drug, but we need better markers for differential responses between people."
Moving forward, the goal is to test rapamycin as a therapy for human conditions of aging such as cardiomyopathies, muscle failure, dementia and cognitive decline.
Another Approach
Alumnus Alessandro Bitto, PhD '13, (meet Alessandro) is also studying rapamycin, the mTOR pathway and aging. A molecular pathobiologist and an acting instructor at the University of Washington, Bitto first became interested in this area of research while working in Sell's laboratory. He now looks at rapamycin for the treatment of mitochondrial disease and obesity, and what can be learned about aging vis-à-vis changing metabolic processes.
"I'm trying to develop the concept that obesity and metabolic complications also have common factors with normal aging; rapamycin and other drugs are effective in both situations," he says.
One of the intriguing qualities of rapamycin is that it mimics the effects of caloric restriction, which has been shown to prolong life.
A frequent collaborator with Sell, Eishi Noguchi, PhD, an associate professor of biochemistry and molecular biology, is using rapamycin to replicate the effects of a calorie-restricted diet through the mTOR pathway.
"We know that restricting calories has been good for health and extending life, but we want to understand exactly what mechanisms are at work — why eating fewer calories prevents DNA damage," he says.
His lab is looking at the mTOR pathway that is central to cell growth, proliferation and metabolism. In calorie-restricted diets, mTOR is inhibited and its direct target Maf1 is activated. In his recent report, he investigated the role of Maf1 in extending lifespan under calorie restriction. Closer examination of Maf1's role in protecting against DNA deterioration could allow Noguchi and his colleagues to specifically target Maf1. Ultimately, that could be the basis for the development of a drug that improves lifespan with fewer side effects than a less targeted treatment.
"The maximum lifespan right now is around 120 years old, and at that point most people are sick," says Noguchi. "We are not necessarily looking to have people live longer but to extend the health span as they age.
"If one day we can control our DNA and cellular activities to make them fully functional, we can improve people's lives."
The DNA Puzzle
Biochemist Kate Beishline, PhD '13, worked in the lab of Jane Azizkhan-Clifford, PhD, professor and chair of biochemistry and molecular biology, while she was at Drexel studying DNA damage and repair. Now a faculty fellow and assistant professor at Bloomsburg University, she is studying the pathways of cellular aging at the molecular level, specifically CTCF (chromatin insulator protein CCCTC binding factor) and its role in telomere stability in normal cells and cancer cell formation.
"For a long time, people were focusing on the length of telomeres as the holy grail of aging, but it's not that simple," she says. "Ultimately, I would like to better understand DNA methylation and its relationship with CTCF and telomeres, as DNA methylation can be highly affected by aging cells."
Beishline didn't initially plan to study aging, but developed an interest in the subject through her work on genomic stability. She is not as interested in finding an intervention for aging or its biological processes as in simply defining its mechanisms.
"I like looking at these pieces and understanding the pathways rather than focusing on the bigger picture. To me it's just about the curiosity to answer this question and then the next one," she says. "If we know more about how telomere length is regulated in normal cells, for instance, we might have a better understanding of what leads to disease or what makes us age."
Elsewhere in the College of Medicine, researchers are examining aging from the perspective of age-related disease. That includes Elias El Haddad, PhD, a professor of medicine and an immunologist by training.
"One of the biggest health challenges for older people — that means those 65 years and older — is that they have problems responding to vaccines. We know that the flu vaccine won't seroconvert in more than 60 percent of people who are 55 and older. The question is why."

Images of neurons differentiating in culture show how rapamycin (right) enhances this process versus control.
In his lab, El Haddad takes a systems biology approach, looking at older patients' innate immune response from multiple perspectives to identify the immune cells that are unable to respond to pathogens.
"We've found that dendritic cells have altered functions responding to TLR ligands in aging people, which in turn affects their ability to stimulate T cells," he says. "We are now digging further to understand the intercellular defects in each cell and why this is happening. The ultimate objective would be to design a vaccine with an adjuvant molecule that can target these defects."
Such an approach might be applicable in viral diseases like West Nile, yellow fever, smallpox and pneumonia. The stakes, he says, are very high, given the growing aging population and the public health risks of ineffective vaccines. "As more and more people will be over the age of 65, it's important to create a healthy environment."
Healthy Aging and Alzheimer's
Claudio Torres, PhD, an associate professor of neurobiology and anatomy, and microbiology and immunology, has long been interested in the mechanisms of aging that pave the way for neurocognitive disease, including Alzheimer's disease and HIV-related dementia. Key among them is cellular senescence, which can be triggered by DNA damage, oxidative stress, telomere dysfunction and other factors.
Through in vitro cell studies, Torres examines the role of proteins associated with Alzheimer's disease and HIV drugs as inducers of senescence; the consequences of cellular senescence on function of brain cells such as glia and neurons; the analysis of cellular senescence in the human brain during healthy aging and Alzheimer's progression; and pharmacological interventions in senescence. The goal is to ultimately understand these mechanisms clearly enough to develop a way to delay senescence through treatment.
"My laboratory is a pioneer in the study of cellular senescence in the human brain, and we have proposed a central role for glia senescence in the pathogenesis and age-dependency of Alzheimer's disease," Torres says. "There are features of the senescent cells that may explain several signs associated with the disease. For instance, the inflammatory response observed in Alzheimer's patients could be explained by similar inflammatory factors highly produced and secreted by aging astrocytes, which vastly populate the brain of these patients.
"Age is the major risk factor for Alzheimer's, and our research supports cellular senescence as the mechanism that links aging with this pathology. Perhaps even more important, our research suggests that brain-targeted interventions in the senescence program may ameliorate the initiation and progression of Alzheimer's disease.
"We've seen that we can eliminate senescent brain cells, and other labs have shown in mouse models that behavior and cognition improve. This could have broader implications for delaying the onset of other diseases, and it has been very promising so far," Torres says.
We have a unique situation at Drexel, where we can collaborate as a team across departments and schools.
At the University level, the Cell2Society Aging Research Network, a one-year initiative focused on use-inspired aging research, brings together 23 faculty members with wideranging expertise from a variety of disciplines, including biology, nursing (the PI and others), medicine, biomedical engineering and public health, among others. Sell is a member of the steering committee, and surgeon Michael Weingarten, MD, is a member of the research network.
Weingarten has two ongoing studies that could help improve the health of older patients. In one, the professor is testing the use of low-frequency ultrasound to heal chronic wounds, a major health risk in the aging population, particularly for diabetes patients. Another study is looking at the use of infrared technology for earlier detection of pressure ulcers in immobilized patients.
"We have a unique situation at Drexel, where we can collaborate as a team across departments and schools to do research like this," Weingarten says.
More PhD students are coming to Drexel planning to conduct research related to aging, according to Noguchi, who directs the Molecular & Cell Biology & Genetics graduate program. With the aging of the population at large, the field will continue to grow.
"It's exciting to think about the continuum of work here, from basic biology to immune function to the clinical side, and interventions that can help prevent age-related health conditions," Sell says. "This is one of the last frontiers in biology. We are not designed to go on forever. When I first started in science, the idea of intervening in aging seemed impossible — and yet here we are."
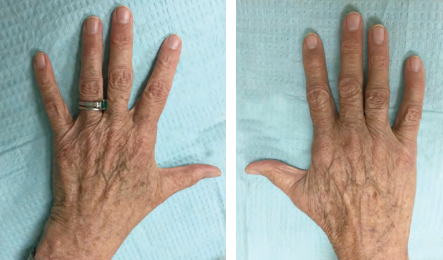
Comparison of the placebo-treated hand at left to the rapamycintreated hand shows that clinical signs of aging were improved in the treated hand.
How It Works
Basic science studies have used rapamycin to slow aging in mice, flies, and worms, but the study led by Christian Sell, PhD, is the first to show an effect on aging in human tissue.
"We were seeing a growing potential for use of this drug, so we said, let's try skin," Sell explained. "It's a complex organism with immune, nerve cells, stem cells — you can learn a lot about the biology of a drug and the aging process by looking at skin."
In the trial, 13 participants over age 40 applied rapamycin cream every 1-2 days to one hand and a placebo to the other hand for 8 months. The researchers checked on subjects after 2, 4, 6 and 8 months, including conducting a blood test and a biopsy at the 6- or 8-month mark.
After 8 months, the majority of the rapamycin hands showed increases in collagen protein, and statistically significant lower levels of p16 protein, a key marker of skin cell aging. Skin that has lower levels of p16 has fewer senescent cells, which are associated with skin wrinkles. Beyond cosmetic effects, higher levels of p16 can lead to dermal atrophy, which is associated with fragile skin that tears easily, slow healing after cuts and increased risk of infection or complications after injury.
The capability for rapamycin to improve human health beyond outward appearance is further illuminated when looking deeper at p16 protein, which is a stress response that human cells undergo when damaged but is also a way of preventing cancer. When cells have a mutation that would have otherwise created a tumor, this response helps prevent the tumor by slowing the cell cycle process. Instead of creating a tumor, it contributes to the aging process. "These cells that have undergone stress are now pumping out inflammatory markers."
In addition to its FDA-approved use to prevent organ rejection, rapamycin is prescribed (in higher doses than used in the current study) for the rare lung disease lymphangioleiomyomatosis and as an anti-cancer drug. The current Drexel study shows a second life for the drug in low doses, including new applications for studying rapamycin to increase human lifespan or improve human performance.
There are two pending patents on this technology, both of which have been licensed to Boinca Therapeutics LLC, of which Sell and another author are shareholders.
— Greg Richter
*Christina Lee Chung, Ibiyonu Lawrence, Melissa Hoffman, Dareen Elgindi, Kumar Nadhan, Manali Potnis, Annie Jin, Catlin Sershon, Rhonda Binnebose, Antonello Lorenzini, and Christian Sell: "Topical Rapamycin Reduces Markers of Senescence and Aging in Human Skin: An Exploratory, Prospective, Randomized Trial" in GeroScience Volume 41, Issue 6, December 2019 (online November 25, 2019, doi.org/10.1007/s11357-019-00113-y).
Back to Top