Synthesis in DNI falls into 3 major categories:
1. Synthesis of
new MXenes through MAX synthesis.
Typically, MXenes are synthesized through a topochemical selective etching
process of MAX (Mn+1AXn) phase materials, where M is an
early transition metal (Ti, V, Cr, Nb, etc.) A is Al, Ga, Si, S, etc., and X is
carbon and/or nitrogen, and n is 1-3.1 By synthesizing MAX phase
materials (Ti3AlC2, V2AlC, Nb4AlC3,
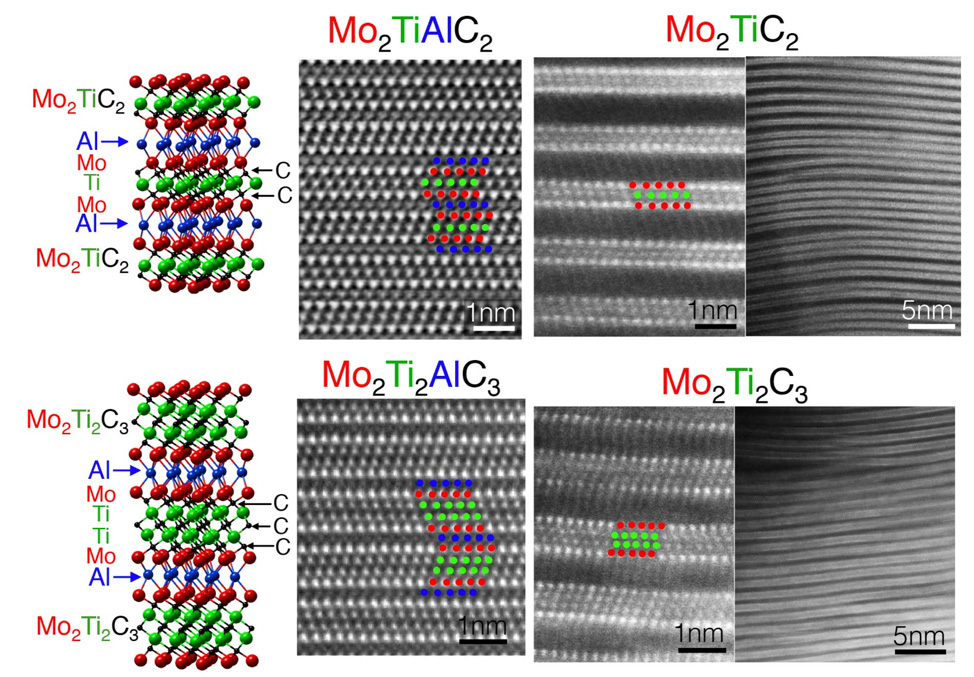
Mo2Ti2AlC3, etc.) their corresponding MXenes
(Ti3C2Tx, V2CTx,
Nb4C3Tx, Mo2Ti2C3Tx,
etc.) can be synthesized.2-4 Each aspect of MXene
chemistry/structure including n (Ti2CTx vs. Ti3C2Tx),
M (V2CTx vs. Ti2CTx),
and X (Ti3C2Tx vs. Ti3CNTx)
affects the resulting properties and applications.5 Within MXenes, there are X general
classes synthesized to date: single M MXenes (Ti3C2Tx),
ordered-double transition metal MXenes (Mo2Ti2C3Tx),2 solid-solution MXenes (Ti2-xVxCTx),
and ordered divacancy MXenes (Mo1.33CTx).6 Thus far, over 30 MXenes have been
discovered, with the potential for hundreds more.
2. New
synthetic routes to etch MXenes.
When MXenes were first discovered, the method of production was the use of high
concentration HF.7 This HF would interact with the Al
layer in MXenes, selectively removing them, while leaving the Mn+1Xn
structure intact. This approach leads to the characteristic accordion-like
structure of MXenes. In today’s language, we call this multilayer MXene.
Afterwards, high-intensity sonication was carried out, leading to delamination
MXene, with sizes in submicron range. After this initial discovery, different
concentrations of HF were used, and this led to lower quantities of defects in
the initial MXenes, and thus better properties. At this stage, a variety of
intercalants (TMAOH, DMSO, etc.) were found to chemically delaminate the MXene
flakes, rather than mechanically. A new method was discovered, termed the MILD
method, that used a combination of HCl+LiF to produce HF in-situ and
simultaneously delaminate the MXene by intercalating lithium ions into the
structure.8 Since then, a new approach was
developed using a combination of HF+HCl, followed by a separate delamination
step with LiCl. These advances led to progressively larger and larger MXenes
(now >10 μm). In addition to increasing optimize methods for removal of
Al, there have been advances in selectively moving other A elements, such as Si
or Ga.9-10
3. Nitridation
of MXenes. The
electronic properties of nitride-based MXenes were studied computationally, and
it was found that they should possess higher stability and electrical
conductivity, among other enhanced properties. However, synthesis of the MAX
phase precursors is a challenge; there has been successful reports of only a
few nitride MAX phases. Considering this, nitride materials are more
thermodynamically favorable, thus, with appropriate conditions, it is possible
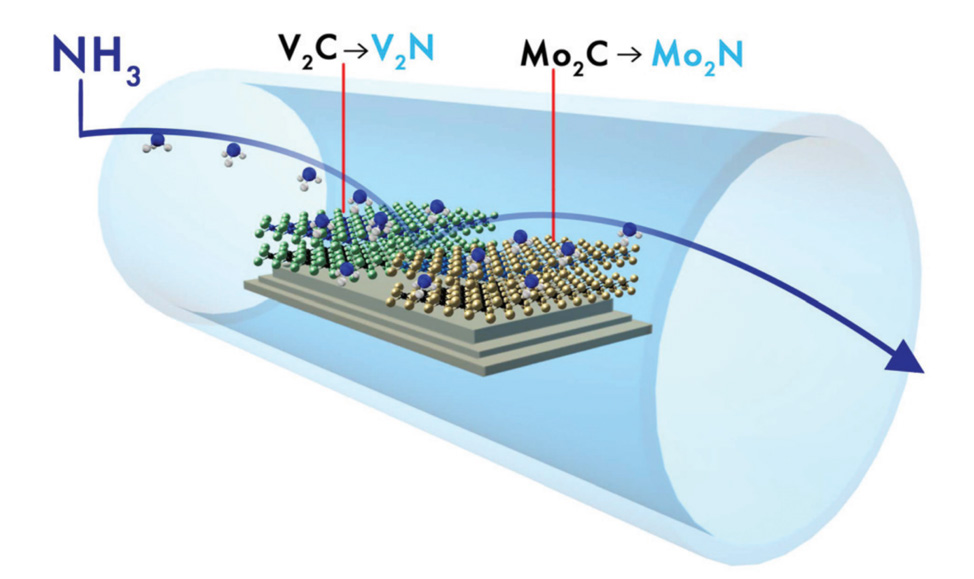
to chemically convert carbide MXenes into nitride MXenes. Previously, we have
utilized NH3 gas at high temperatures (600°C) to convert Mo2CTx
and V2CTx into their nitridized forms, Mo2NTx
and V2NTx, respectively.11 These nitride MXenes had three
orders of magnitude higher electrical conductivity than their respective
carbide forms. Using a similar approach, we have also converted salt-template
grown oxides into 2D nitrides.12-13
Five Top Papers:
- Anasori,
B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B. C.; Hultman, L.; Kent, P. R. C.;
Gogotsi, Y.; Barsoum, M. W., Two-Dimensional, Ordered, Double Transition Metals Carbides (MXenes). ACS Nano 2015, 9 (10) 9507-9516.
- Alhabeb,
M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y.,
Guidelines for Synthesis and Processing of 2D Titanium Carbide (Ti3C2Tx
MXene). Chem. Mater. 2017, 29 (18), 7633-7644.
- Urbankowski,
P.; Anasori, B.; Hantanasirisakul, K.; Yang, L.; Zhang, L.; Haines, B.; May,
S.; Billinge, S. J. L.; Gogotsi, Y., 2D Molybdenum and Vanadium Nitrides Synthesized by Ammoniation of 2D Transition Metal Carbides (MXenes). Nanoscale 2017,
9, 17722-17730.
- Sokol,
M.; Natu, V.; Kota, S.; Barsoum, M. W., On the Chemical Diversity of the MAX Phases. Trends Chem. 2019, 1 (2) 210-223.
- Xiao,
X.; Wang, H.; Urbankowski, P.; Gogotsi, Y., Topochemical Synthesis of 2D Materials. Chem. Soc. Rev. 2018, 47 (23), 8744-8765.
References
- Sokol,
M.; Natu, V.; Kota, S.; Barsoum, M. W., On the Chemical Diversity of the MAX
Phases. Trends Chem. 2019, 1 (2) 210-223.
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu,
J.; Hosler, B. C.; Hultman, L.; Kent, P. R. C.; Gogotsi, Y.; Barsoum, M. W.,
Two-Dimensional, Ordered, Double Transition Metals Carbides (MXenes). ACS
Nano 2015, 9 (10) 9507-9516.
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.
M.; Hultman, L.; Gogotsi, Y.; Barsoum, M. W., New Two-dimensional Niobium and Vanadium
Carbides as Promising Materials for Li-ion Batteries. J. Am. Chem. Soc. 2013,
135 (43), 15966-15969.
- Ghidiu, M.; Naguib, M.; Shi, C.;
Mashtalir, O.; Pan, L. M.; Zhang, B.; Yang, J.; Gogotsi, Y.; Billing, S. J. L.;
Barsoum, M. W., Synthesis and Characterization of Two-Dimensional Nb4C3
(MXene). Chem. Commun. 2012, 50, 9517-9520.
- Hantanasirisakul, K.; Alhabeb, M.;
Lipatov, A.; Maleski, K.; Anasori, B.; Salles, P.; Ieosakulrat, C.;
Pakawatpanurut, P.; Sinitskii, A.; May, S. J., Effects of Synthesis and
Processing on Optoelectronic Properties of Titanium Carbonitride MXene. Chem.
Mater. 2019, 31 (8), 2941-2951.
- Tao, Q.; Dahlqvist, M.; Lu, J.; Kota,
S.; Meshkian, R.; Halim, J.; Palisaitis, J.; Hultman, L.; Barsoum, M. W.;
Persson, P. O. A.; Rosen, J., Two-dimensional Mo1.33C MXene with Divacancy
Ordering Prepared from Parent 3D Laminate with In-plane Chemical Ordering. Nat.
Commun. 2017, 8, 14949.
- Naguib, M.; Mashtalir, O.; Carle, J.;
Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M. W., Two-dimensional Transition
Metal Carbides. ACS Nano 2012, 6 (2), 1322-31.
- Alhabeb, M.; Maleski, K.; Anasori, B.;
Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y., Guidelines for Synthesis and
Processing of 2D Titanium Carbide (Ti3C2Tx
MXene). Chem. Mater. 2017, 29 (18), 7633-7644.
- Alhabeb, M.; Maleski, K.; Mathis, T.
S.; Sarycheva, A.; Hatter, C. B.; Uzun, S.; Levitt, A.; Gogotsi, Y., Selective
Etching of Silicon from Ti3SiC2 (MAX) Produces 2D
Titanium Carbide (MXene). Angew. Chem. Int. Ed. 2018, 130
(19), 5542-5546.
- Meshkian, R.; Näslund, L.-Å.; Halim, J.;
Lu, J.; Barsoum, M. W.; Rosen, J., Synthesis of two-dimensional molybdenum
carbide, Mo2C, from the gallium based atomic laminate Mo2Ga2C. Scripta
Materialia 2015, 108, 147-150.
- Urbankowski, P.; Anasori, B.;
Hantanasirisakul, K.; Yang, L.; Zhang, L.; Haines, B.; May, S.; Billinge, S. J.
L.; Gogotsi, Y., 2D Molybdenum and Vanadium Nitrides Synthesized by Ammoniation
of 2D Transition Metal Carbides (MXenes). Nanoscale 2017, 9,
17722-17730.
- Xiao, X.; Urbankowski, P.;
Hantanasirisakul, K.; Yang, Y.; Sasaki, S.; Yang, L.; Chen, C.; Wang, H.; Miao,
L.; Tolbert, S. H., Scalable Synthesis of Ultrathin Mn3N2
Exhibiting Room‐Temperature Antiferromagnetism. Adv. Funct. Mater. 2019,
29 (17), 1809001.
- Xiao, X.; Wang, H.; Urbankowski, P.;
Gogotsi, Y., Topochemical Synthesis of 2D Materials. Chem. Soc. Rev. 2018,
47 (23), 8744-8765.