Titanium dioxide (TiO2) is one of the most versatile oxides known with a wide range of applications from biomedical uses, to paints, to electronics and many more. When processed at the nanoparticle scale, the uses expand even further to include consumer products ranging from energy storage to cosmetics and more. This popularity stems from its whiteness, ubiquity, low cost, chemical stability, biocompatibility and non-toxicity. Despite their popularity, making nanostructured titanium dioxides is often slow and/or hazardous and, as a result, bulk production is expensive and time consuming.
Recently, Distinguished Professor Michel Barsoum and members of his Layered Solids Group reported on their discovery of a solution based, near-ambient method for producing one dimensional (1D) TiO2 nanofilaments (NFs) at the kilogram scale, starting with inexpensive, non-toxic precursors. Structurally these 1D NFs are quite remarkable. “It is the thinnest spaghetti ever made,” Barsoum says. “If the NFs in one gram of their 1D material were laid end-to-end they would span 400 million miles!”
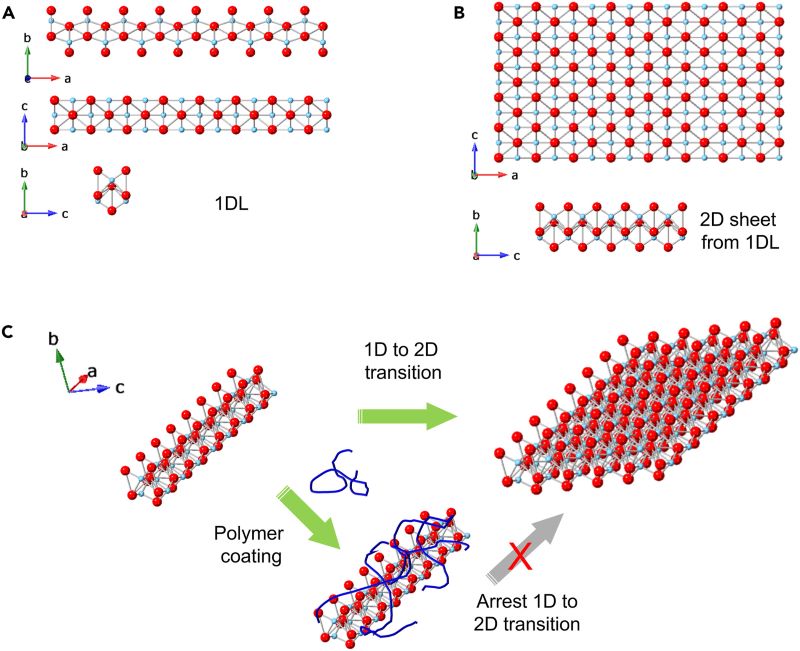
As produced, the 1D NFs exist as a colloidal suspension; think spaghetti in a pot of water. When the water is drained from the pot, the 1D NFs self-assemble into two dimensional (2D) layers that are stacked like lasagna. When Barsoum’s group investigated the 1D NFs in this state via microscopy, and X-ray diffraction, they were able to determine their approximate lengths and thicknesses, but they could not tell how wide each strand was. Taking the food analogy one step further; it was unclear whether these 2D layers were made up of spaghetti or linguine. This was a problem because having a complete understanding of their structure is key to being able to fully investigate 1D NF’s properties, performance and potential applications.
Professor Christopher Li, head of the Soft Materials Group, offered a potential solution to Barsoum’s dilemma. He suggested that wrapping a polymer coating around each 1D NF in its original 1D state could prevent it from transitioning to 2D sheets, allowing them to fully characterize its structure. His suggestion worked and the result was unambiguous; in the colloid state they concluded they had made the thinnest spaghetti-like structure ever made. Li and Barsoum then called upon assistant professor Yong-Jie Hu who leads the Materials Computation and Informatics Group, who provided modeling and simulation to help better understand 1D NFs atomic structure and the differences between its 1D and 2D states.
The team successfully prevented the NFs from transforming to 2D sheets by wrapping every individual 1D NF with an atomically thick layer of polymer. As an unexpected bonus, the polymer coated NFs crystallized into a honeycomb-like columnar hexagonal structure. This not only allowed the team to fully characterize the structure of NFs in the colloidal state, but they could now control the distance between 1D NFs in the hexagonal arrangement by simply varying the amount of polymer added to the colloidal suspension. This control provides tunability; by altering the distance between NFs at the atomic scale it is possible to control properties and present opportunities for even more 1D NF applications. “In the materials field, we try to control structure to give us certain properties,” notes Barsoum. “But it’s a very difficult thing to control at the nanoscale. This is a quite elegant, simple, cost-effective and scalable way of doing that.”
The team’s findings were reported in “Tuning the 1D-to-2D transition in lepidocrocite titanate nanofilaments via polymer wrapping” in the journal Matter.