
After nearly a decade of research showing that MXene materials can be used to improve a variety of technology, Drexel researchers now have a way to make the material in large enough batches to be considered viable for manufacturing.
For more than a decade, two-dimensional nanomaterials, such as graphene, have been touted as the key to making better microchips, batteries, antennas and many other devices. But a significant challenge of using these atom-thin building materials for the technology of the future is ensuring that they can be produced in bulk quantities without losing their quality. For one of the most promising new types of 2D nanomaterials, MXenes, that’s no longer a problem. Researchers at Drexel University and the Materials Research Center in Ukraine have designed a system that can be used to make large quantities of the material while preserving its unique properties.
The team recently reported in the journal Advanced Engineering Materials that a lab-scale reactor system developed at the Materials Research Center in Kyiv, can convert a ceramic precursor material into a pile of the powdery black MXene titanium carbide, in quantities as large as 50 grams per batch.
Proving that large batches of material can be refined and produced with consistency is a critical step toward achieving viability for manufacturing. For MXene materials, which have already proven their mettle in prototype devices for storing energy, computing, communication and health care, reaching manufacturing standards is the home stretch on the way to mainstream use.
“Proving a material has certain properties is one thing, but proving that it can overcome the practical challenges of manufacturing is an entirely different hurdle — this study reports on an important step in this direction,” said Yury Gogotsi, PhD, Distinguished University and Bach professor in Drexel’s College of Engineering, who has pioneered the research and development of MXene and is a lead author of the paper. “This means that MXene can be considered for widespread use in electronics and energy storage devices.”
Researchers at Drexel have been making MXene in small quantities — typically one gram or less — since they first synthesized the material in 2011. The layered nanomaterial, which looks like a powder in its dry form, starts as a piece of ceramic called a MAX phase. When a mixture of hydrofluoric and hydrochloric acid interacts with the MAX phase it etches away certain parts of the material, creating the nanometer-thin flakes characteristic of MXenes.
In the lab, this process would take place in a 60 ml container with the ingredients added and mixed by hand. To more carefully control the process at a larger scale, the group uses a one-liter reactor chamber and a screw feeder device to precisely add MAX phase. One inlet feeds the reactants uniformly into the reactor and another allows for gas pressure relief during the reaction. A specifically designed mixing blade ensures thorough and uniform mixing. And a cooling jacket around the reactor lets the team adjust the temperature of the reaction. The entire process is computerized and controlled by a software program created by the Materials Research Center team.
The group reported successfully using the reactor to make just under 50 grams of MXene powder from 50 grams of MAX phase precursor material in about two days (including time required for washing and drying the product). And a battery of tests conducted by students at Drexel’s Materials Science and Engineering Department showed that the reactor-produced MXene retains the morphology, electrochemical and physical properties of the original lab-made substance.
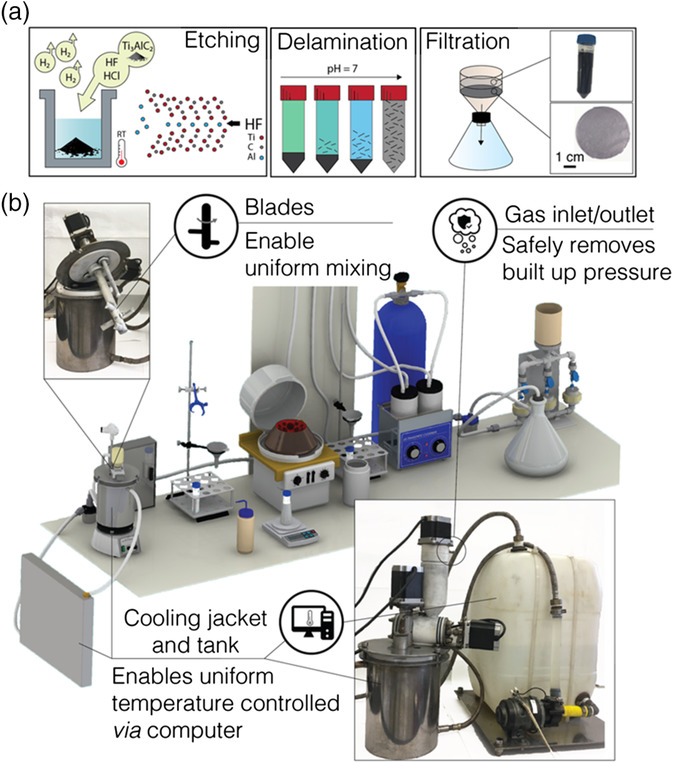
The reactor system tested by Drexel researchers can produce as much as 50 grams of MXene material at a time.
This development puts MXenes in a group with just a handful of 2D materials that have proven they can be produced in industrial-size quantities. But because MXene-making is a subtractive manufacturing process — etching away bits of a raw material, like planing down lumber —
it stands apart from the additive processes used to make many other 2D nanomaterials.
“Most 2D materials are made using a bottom-up approach,” said Christopher Shuck, PhD, a post-doctoral researcher in the A.J. Drexel Nanomaterials Institute. “This is where the atoms are added individually, one by one. These materials can be grown on specific surfaces or by depositing atoms using very expensive equipment. But even with these expensive machines and catalysts used, the production batches are time-consuming, small and still prohibitively expensive for widespread use beyond small electronic devices.”
MXenes also benefit from a set of physical properties that ease their path from processed material to final product — a hurdle that has tripped up even today’s widely used advanced materials.
“It typically takes quite a while to build out the technology and processing to get nanomaterials in an industrially usable form,” Gogotsi said. “It’s not just a matter of producing them in large quantities, it often requires inventing completely new machinery and processes to get them in a form that can be inserted into the manufacturing process — of a microchip or cell phone component, for example.”
But for MXenes, integrating into the manufacturing line is a fairly easy part, according to Gogotsi.
“One huge benefit to MXenes is that they be used as a powder right after synthesis or they can be dispersed in water forming stable colloidal solutions,” he said. “Water is the least expensive and the safest solvent. And with the process that we’ve developed, we can stamp or print tens of thousands of small and thin devices, such as supercapacitors or RFID tags, from material made in one batch.”
This means it can be applied in any of the standard variety of additive manufacturing systems — extrusion, printing, dip coating, spraying — after a single step of processing.
Several companies are looking developing the applications of MXene materials, including Murata Manufacturing Co, Ltd., an electronics component company based in Kyoto, Japan, which is developing MXene technology for use in several high-tech applications.
“The most exciting part about this process is that there is fundamentally no limiting factor to an industrial scale-up,” Gogotsi said. “There are more and more companies producing MAX phases in large batches, and a number of those are made using abundant precursor materials. And MXenes are among very few 2D materials that can be produced by wet chemical synthesis at large scale using conventional reaction engineering equipment and designs.”
Read the full paper here: https://onlinelibrary.wiley.com/doi/abs/10.1002/adem.201901241
This research was supported by the U.S. Office of the Director of National Intelligence, the National Science Foundation, the European Commission and the U.S. Department of Energy.
In addition to Gogotsi and Shuck, Asia Sarycheva, Mark Anayee, Ariana Levitt, Yuanzhe Zhu and Simge Uzun, from Drexel; and Vitaliy Balitskiy, Veronika Zahorodna and Oleksiy Gogotsi, from the Materials Research Center, participated in this research.