Research in the Romano lab is focused on studying cancer drug resistance using a conjunction of mouse modeling, functional genomics and computational biology. We study intrinsic and acquired molecular mechanisms by which tumor cells resist targeted and/or immunotherapy approaches, with the final aim of developing novel therapeutic strategies for cancer patients. We are particularly interested in:
View Romano Lab Publications
View Romano Lab Members
The tumor-stroma immunological axis in minimal residual disease
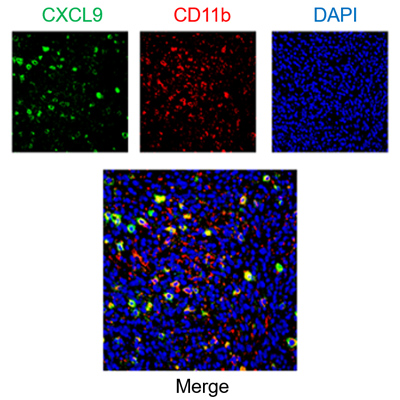
Multiplex staining of immune cells and chemokines in mouse tumor samples.
When a small group of drug-tolerant cells evades treatment, it can give rise to a Minimal Residual Disease, where tumor cells linger in a dormant state. As Minimal Residual disease can fuel relapse and metastasis, its eradication is fundamental to obtaining long-lasting clinical effects. We are currently optimizing chemokine-based approaches to influence the composition of the immune infiltrate and direct the immune system toward eliminating residual tumor cells.
The role of tumor suppressors in cancer drug resistance
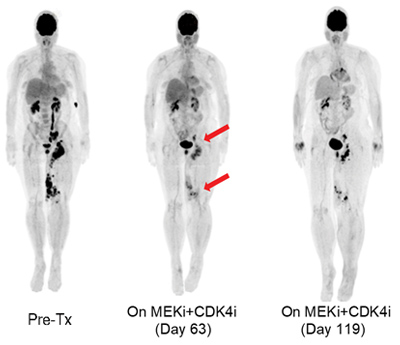
PET-scan of a metastatic melanoma patient who initially responded to therapy but eventually developed resistance.
Most of the current targeted therapy approaches inhibit oncogene activity (i.e., BRAF inhibitors, EGFR inhibitors), often obtaining impressive clinical responses. Nevertheless, cancer is a genetic illness where at least 2-3 tumor suppressor gene mutations are usually required for tumorigenesis. Our lab uses functional genomics experiments to detect and anticipate tumor-suppressor-mediated resistance mechanisms to targeted therapy. We aim to develop effective counter-therapies to administer in conjunction or substitution of the current standard of care approaches.
Immunological mechanisms of HIV and cancer co-morbidity
People living with HIV (PLWH) are more prone to develop cancer due to their lowered immune surveillance. Although antiretroviral therapy has sharply decreased the prevalence of AIDS-defining cancers, there has been an increased incidence of non-AIDS-defining cancers. Importantly, PLWH who develop cancer show a significantly higher mortality rate than uninfected people. PLWH are generally less immunocompetent at a basal level, and evidence suggests that antiretroviral therapy can deplete T-cells in the tissues of specific subtypes of patients. Consequently, PLWH are at a major risk of developing an incomplete response to therapy and eventually relapsing. We aim to develop adjuvant treatment strategies to anticipate drug resistance and improve the outcome of cancer treatment in PLWH.
The Romano lab has experience in:
- Mouse models of cancer (GEMM, xenografts, syngeneic)
- Biological validation of targets through functional genomics (knock-in, knock-out, CRISPR, Cre-LoxP)
- Drug and genetic screening for the identification of actionable targets of drug sensitivity (CRISPR screens, chemical library screens)
- Micro- and nano-particle delivery of immunomodulating compounds
- Bioinformatic analysis of "omic" data (RNAseq, WES, RPPA) on patient and mouse samples
- Collaborations across the US and global institutions for the coordinated development of translational projects

Bioinformatic deconvolution of tumor immune infiltrate before and after treatment.
Open Position
Students (grad and undergrad) and potential collaborators are always welcome to contact Dr. Romano for questions, ideas, and suggestions!
Back to Top