Research
One goal of our research is to enhance our understanding of how drugs of abuse, antidepressants, and other small molecules interact with and modulate the function of monoamine transporters (MATs), including the serotonin transporter (SERT), the dopamine transporter (DAT), and the norepinephrine transporter (NET). DAT, NET, and SERT serve a pivotal role in limiting monoamine-mediated neurotransmission through the reuptake of their respective neurotransmitters, and the continuing need for therapeutic drugs to treat brain disorders involving aberrant monoamine signaling provides a compelling reason to further our understanding of transporter function and to identify novel ways of targeting them.
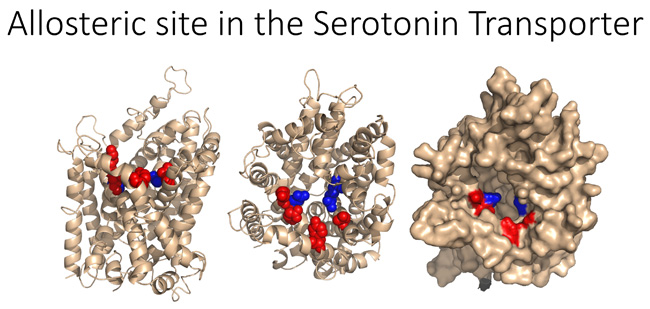
We have recently isolated SERT and DAT cDNAs from the human parasite Schistosoma mansoni. The uptake kinetics for serotonin are indistinguishable between human and parasite SERT, but the parasite SERT and DAT displays dramatically reduced affinity for amphetamines and several inhibitors including cocaine and SSRIs. These results suggest that subtle structural differences between the parasite and human carrier exist that determine exogenous substrate and inhibitor interactions. We have pursued this idea and identified a novel allosteric site in the MATs, and we discovered novel compounds that interact with this site and display unique functional properties. Our lab is employing structure/function studies, molecular modeling, and in silico virtual drug screens to further pursue this avenue of research. This project is being developed in collaboration with Dr. Sandhya Kortagere in Microbiology & Immunology.
We believe this project will lead to important insights into novel ways of regulating monoamine transporter action. We specifically expect to identify molecules that enhance transporter function and alter the interaction between the transporters and their classical ligands, including psychostimulants and antidepressants. The work will also supply probes that can provide mechanistic information about monoamine transporter function. Importantly, this work will position us to better understand how novel allosteric sites within the MATs can be employed as a therapeutic target that could lead to more effective treatment of mental disorders that involve monoamine signaling, including depression, ADHD, schizophrenia, impulse control disorders, and drug and alcohol abuse.
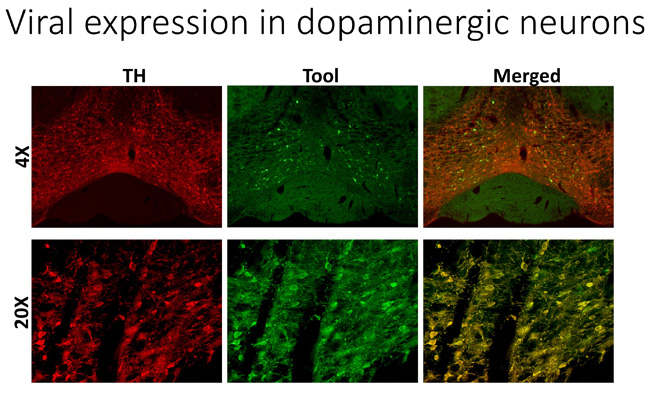
In an unrelated project, we are developing and employing state-of-the-art, viral-based tools to establish the role of intracellular signaling in distinct neuronal populations in the brain to investigate how this signaling is involved in adaptive and mal-adaptive changes. The brain continuously adapts and responds to external stimuli by activating intracellular signaling cascades that produce long-term molecular changes. These long-term adaptive molecular changes in the brain are important mediators of tuning and adjusting brain function in processes that involve learning and memory, as one example, but also in maladaptive processes as in drug addiction. Previous studies in this field have been limited because tools that target discrete neuronal populations in vivo do not currently exist, and the previous studies were performed in vitro, ex vivo, or in animals systemically affected by the specific manipulations.
To overcome this limitation and achieve our goals, we are designing unique genetic tools that are designed to only target specific types of brain cells. We believe this work will lead to novel insights into signaling systems that mediate adaptive cellular and molecular changes within specific neurons such as dopaminergic neurons. We will use this work to establish how these changes are involved in the development of cocaine addiction, for example. We anticipate that this will facilitate the identification of novel targets that mediate cocaine-associated behaviors and believe this potentially will lead to novel therapeutic avenues for treating this disorder. This project is being developed in collaboration with Dr. Rodrigo España in Neurobiology & Anatomy.
The potential of the strategy we are employing is not limited to studies of drug addiction, and our studies will eventually be expanded to understand how intracellular signaling in particular neuronal populations contribute to various disease states of the brain including drug addiction, psychiatric disorders, and neurodegenerative diseases such as Parkinson's, Alzheimer's and Huntington's.
Back to Top