 Fafarman
Fafarman
When scientists study how materials behave under extreme conditions, they
typically examine what happens under compression. But what occurs when you
pull matter apart in all directions simultaneously? This phenomenon, known
as "negative pressure," has largely remained theoretical - until now.
A team of researchers at Drexel University has developed a method to achieve
and precisely control negative pressure in crystals, opening new
possibilities for engineering materials with unique properties. The
research, published in the
Proceedings of the National Academy of Sciences
, demonstrates the first experimental platform for studying how crystal
structures respond when systematically and uniformly stretched rather than
compressed.
"Externally applied pressure is a powerful means to control the crystal
structure of solids," said
Aaron Fafarman, PhD
, associate professor of chemical and biological engineering. "Until now,
researchers have been limited to testing how crystal structures vary under
greater and greater pressure, but there has not been a way to perform the
opposite experiment: to continuously stretch the material equally in three
dimensions, rather than compressing it."
The researchers achieved this by synthesizing perovskite crystals within the
nanoscopic pores of a rigid aluminum oxide scaffold at high temperatures.
Fafarman explains the process using a familiar analogy: "It's similar to
cooking a hard-boiled egg. The egg whites solidify in a thermally expanded
state, bonding with the hard, unmoving shell at the cooking temperature.
Once the egg returns room temperature, that bond provides a force that keeps
the whites expanded. If you were to crack the egg, the force (negative
pressure) exerted by the shell would be released, causing the shell to
collapse inward as the whites contract. The key difference is that we do not
crack the shell (/scaffold), thus preserving the negative pressure acting on
the perovskite crystals."
 Chakrabarti
Chakrabarti
In experiments primarily performed by Dr. Arkita Chakrabarti, then a PhD
student in Fafarman’s lab, it was found that this method could generate
negative pressures of hundreds of megapascals (with one megapascal being ten
times atmospheric pressure) simply by varying the synthesis temperature.
When confined within pores smaller than 40 nanometers, the crystals
experienced uniform negative pressure that could be precisely controlled.
This threshold is crucial, Fafarman notes: "Forces exerted by the walls will
necessarily dissipate as you move away from the interface between scaffold
and guest material. If the pore size is large, most of the guest material is
far away from the interface and not experiencing a large force, if any."
The research team worked specifically with cesium lead iodide (CsPbI3),
which Fafarman describes as having "ideal properties to demonstrate this
effect and is a technologically intriguing photovoltaic material in its own
right.” Under the conditions studied, the crystal structure became more
symmetrical and organized - a finding that could have significant
implications.
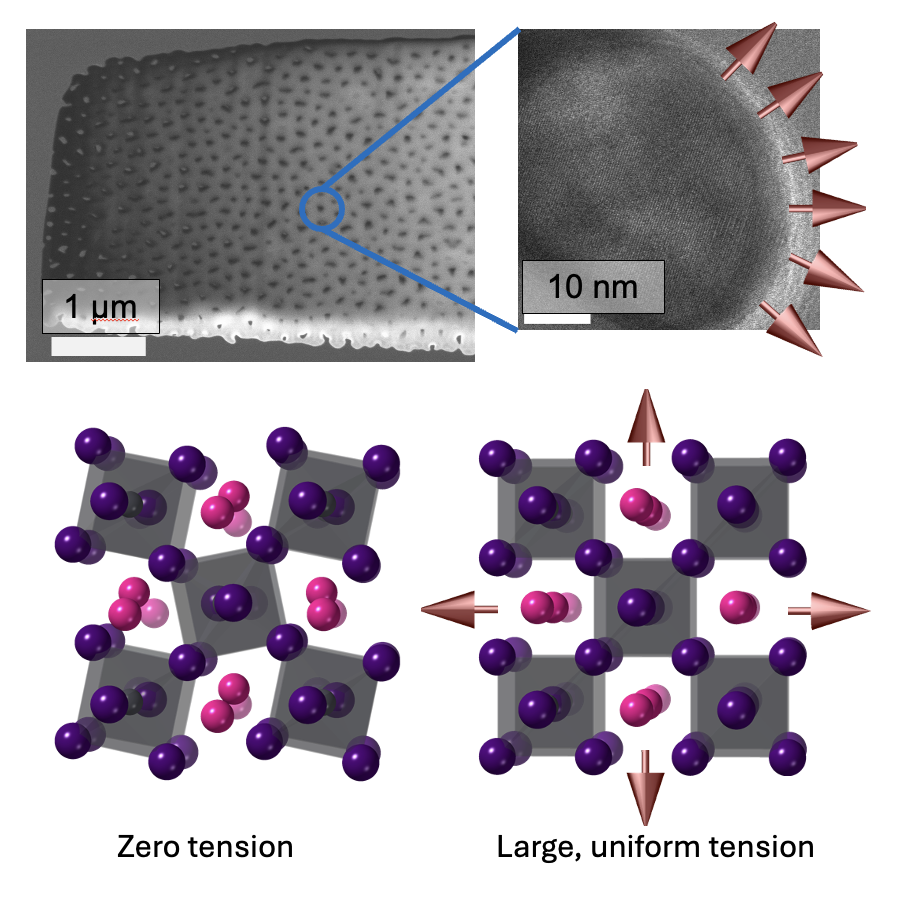 Clockwise from upper left: A electron micrograph of a small segment of the anodized aluminum oxide membrane scaffold, which is filled with the CsPbI3; a close-up image of a single pore of the scaffold, filled with CsPbI3, with arrows indicating the tensile force exerted by the pore walls; a representation of the highly symmetric CsPbI3 crystal structure, achieved when the tensile forces are acting; an example of the natural, low symmetry structure that prevails with no tension.
Clockwise from upper left: A electron micrograph of a small segment of the anodized aluminum oxide membrane scaffold, which is filled with the CsPbI3; a close-up image of a single pore of the scaffold, filled with CsPbI3, with arrows indicating the tensile force exerted by the pore walls; a representation of the highly symmetric CsPbI3 crystal structure, achieved when the tensile forces are acting; an example of the natural, low symmetry structure that prevails with no tension.
"Most mechanical perturbations are symmetry reducing," Fafarman explained.
"Moreover, there are many desirable phenomena in this materials class and
others that accompany higher symmetry, such as absorbing more of the
wavelengths of light in the solar spectrum." However, he suggests the
concept could be applied to other materials with similar properties,
particularly other candidates for photovoltaic applications.
Looking ahead, this breakthrough could open new avenues in materials
science. "Tunable negative pressure is a new handle for exploring
fundamental properties of materials," Fafarman said. "Negative pressure
reduces the interatomic forces outside their familiar range, which could
lead to very different dynamics of atomic motion. For example, facile atomic
motion could enable a phenomenon known as “ferroelectric switching,” with
applications in advanced digital information storage and processing."
The work represents a significant step forward in materials science,
providing the first experimental platform for studying matter under
controlled negative pressure. This fundamental advance could ultimately lead
to new ways of engineering materials with enhanced properties for
applications in electronics, energy conversion, and other fields.