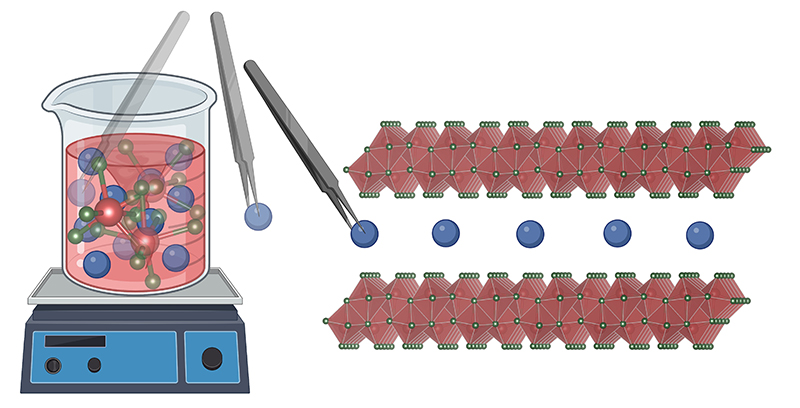
 Illustration of preintercalation synthesis. Credit: Ryan Andris
Illustration of preintercalation synthesis. Credit: Ryan Andris
Ekaterina Pomerantseva, PhD, associate professor in the department of materials science and engineering, has published an overview of the state-of-the-art in chemical preintercalation methods for synthesis of materials for applications in electrochemical energy storage in Accounts of Chemical Research.
Among available types of energy, electricity is currently the most convenient form to transport, store, and use. Balancing the supply and demand of electricity requires storage mechanisms such as batteries and supercapacitors. Additionally, the growth of applications that require autonomous power, such as the Internet of Things and electric vehicles, are dramatically increasing the need for battery storage tailored to various applications. Synthesis methods that can control various qualities of storage materials such as their chemical composition, structure, morphology, and heterointerfaces, while offering versatility and simplicity, are highly desired.
Pomerantseva’s article, “Chemical Preintercalation Synthesis of Versatile Electrode Materials for Electrochemical Energy Storage”, describes a synthesis strategy for the creation of new intercalation host oxides, hybrid materials, and compounds with oxide/carbon heterointerfaces for use as electrodes in intercalation batteries. The chemical preintercalation synthesis process can be used to tune a material’s chemical composition, structure, and morphology. For example, in the case of inorganic ions, preintercalation can be used to improve the ion diffusion and stability of the synthesized materials. In another instance, the article describes how confined interlayer water can be controlled with chemical preintercalation and how the degree of hydration affects the electrochemical performance. And through integration of oxide layers with conductive carbon layers, electronic conductivity of the synthesized electrode materials is drastically improved.
“The chemical preintercalation synthesis approach is already a proven strategy for achieving improved energy storage properties of layered oxide electrodes,” Pomerantseva said. “While we have demonstrated many examples of such superior electrochemical behavior, we are still at the beginning of this journey and are working to show further advances that are enabled by chemical preintercalation method.”
This report encapsulates all of Pomerantseva’s research on chemical preintercalation synthesis to date. Her work has been made possible by grants from the National Science Foundation (DMR-1609272, DMR-1752623, and DMR-2106445) and the Center for Mesoscale Transport Properties, an Energy Frontier Research Center supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, under award no. DE-SC0012673.
Read the full paper here https://pubs.acs.org/doi/full/10.1021/acs.accounts.2c00193