New Protein Structure Holds Answers to PKU
Emilia ("Emily") Arturo, first author of the PKU study, is in the fourth year of her PhD program. Patrick Loll, PhD, is her co-mentor.
College of Medicine researchers are part of an international team of scientists that has made a critical step toward understanding a disorder that affects patients from birth into adulthood.
Phenylketonuria, or PKU, is an inherited metabolic disease that affects one in 20,000 babies born in the United States each year. The enzyme that breaks down the amino acid phenylalanine is either missing or defective in newborns with the disorder. Patients must adhere to a protein-restricted diet throughout their lives to prevent mental and behavioral abnormalities.
But maintaining this strict diet can be a challenge, which intensifies the need for new, non-dietary treatments to combat PKU symptoms.
In a study recently published in Proceedings of the National Academy of Sciences, researchers made significant strides toward that goal by determining the structure of phenylalanine hydroxylase (PAH) — the enzyme that is defective in PKU patients.*
"We have solved the first X-ray crystal structure of full-length PAH," says the study's principal investigator, Eileen K. Jaffe, professor of molecular therapeutics at Fox Chase Cancer Center–Temple Health. "This structure will help us understand the molecular origins of PKU and is an important advance in developing much-needed drugs for patients."
Defects in the PAH enzyme cause phenylalanine to build up to toxic levels in the body. Because nerve cells in the brain are particularly sensitive to phenylalanine levels, excessive amounts of this substance can cause brain defects.
Researchers had tried for decades to determine the structure of the PAH enzyme, says Emilia Arturo, a doctoral student in Drexel's Biochemistry & Molecular Biology program and first author of the study. (Jaffe is Arturo's thesis adviser.)
"Knowing the structure at this high resolution is expected to speed up any current drug discovery pursuits that would otherwise be based only on models of the full-length protein," Arturo says. The research team purified the enzyme and then crystalized the proteins. The researchers ultimately discovered that the PAH enzyme is represented by two distinct structures rather than one. Once the researchers could separate the two, they were able to grow crystals where scientists had not been able to in the past.
Key to understanding the enzyme's structure was the work of Patrick Loll, PhD, a professor in the College of Medicine's Department of Biochemistry & Molecular Biology, who is an expert crystallographer. Arturo grew crystals of the protein that were then exposed to high-intensity X-rays. The scattered radiation from these crystals was collected in the form of images. Together, Loll and Arturo used these images to determine the three-dimensional structure of the protein.
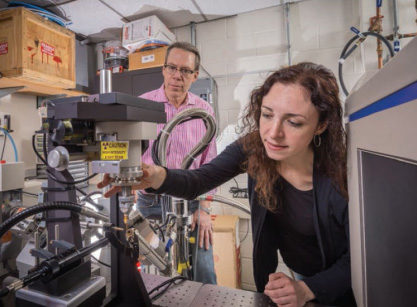
Patrick Loll looks on as Emily Arturo adjusts the X-ray diffraction equipment. The crystal is placed into the high-intensity beam, and the diffraction pattern is recorded by the detector at right.
"Our work was all about visualizing the structure, which is our specialty," says Loll. "Once the research team had figured out how to make the crystals, we joined forces to tell them what was in those crystals, what the enzyme looked like, and how it was arranged."
Using what they have learned from this study, the team is now working to determine the structure of activated PAH, which functions to prevent blood phenylalanine from rising to neurotoxic levels. Knowledge of both PAH structures will help in the use of structure-based drug design techniques to develop medications that will favor activated PAH as a therapeutic approach.
In the end, this research could pave the way for new treatment strategies not only for PKU, but also for cancer and other diseases. Both inborn errors of metabolism and cancer occur when proteins do not function as they should. The research team's focus — how different protein assemblies can be harnessed to control protein function — has tremendous potential for drug discovery, and promises to expand our view of how small molecule therapeutics can work.
* "First Structure of Full-Length Mammalian Phenylalanine Hydroxylase Reveals the Architecture of an Autoinhibited Tetramer" in Proceedings of the National Academy of Sciences, vol. 113, no. 9, March 1, 2016. In addition to researchers mentioned in this article, the study included contributors from the University of Pennsylvania Perelman School of Medicine, Brookhaven National Laboratory, the University of Canterbury and the University of Auckland in New Zealand.
Back to Top