Office of Research & Innovation Newsletter - Winter 2026

In this issue:
An Introduction from Gia Boersema, Assistant Vice Provost, Sponsored Programs
General
- Celebrating and Welcoming ORI Team Members
- Novelution Implementation Update: Upcoming Webinar & User Acceptance Testing Opportunities
- The ORI Annual Report for AY ‘24-‘25 is Now Available!
- Highlighting Drexel News Research Stories: Breakthrough on Gene Therapy for Hereditary Spastic Paraplegia
- Drexel Advances Medical Research with New University City Life Sciences Facility
- Library Support for Open Access Publishing
- New to Altmetric Explorer: Attention Digest Feature
Applied Innovation
Core Faciliities
- Registration for Winter Research Computing Workshops Now Open
- Update on SEM, XRM Trainings at the Materials Characterization Core
Research Compliance & Regulatory Affairs
Sponsored Programs
- Federal Sponsor Updates
- Office of Sponsored Programs Lunch & Learn “How to” Series
- Global Work Requirements for Independent Contractors and Beyond
- New Resource Sheet Helps Researchers Understand Residential Segregation
- Drexel Researchers Receive New Awards!
- Faculty Highlights: Recent Awards and Grants
- Drexel Researchers Awarded AWIS Bridge Grants
- NASA Earth Science Mission Partnerships Calls
Did You Know?
Other Research Training, Education, and Meeting Opportunities
- Register for the Urban Health Summer Institute
- Medicine and Science Leadership Summit - Beyond the Bench: Partnerships and Policy Shaping the Future of Translational Medicine
- LabArchives Love Data Week 2026
Stay Connected with the Office of Research & Innovation!
An Introduction from Gia Boersema, Assistant Vice Provost, Sponsored Programs
As we begin the new year, the Office of Research & Innovation is pleased to share our January 2026 newsletter with the campus research community. This edition marks the start of another year of partnership, service, and shared commitment to advancing the University’s research, scholarship, and innovation mission.
In this issue, we provide key updates from our office, including funding and policy announcements, compliance reminders, upcoming deadlines, and resources available to support faculty and research staff across the full research lifecycle. We also highlight recent accomplishments and initiatives that reflect the breadth and impact of research activity across campus.
As I have transitioned into my role as Assistant Vice Provost for Sponsored Programs over the past several months, I have been consistently impressed by the dedication, resilience, and adaptability of Drexel’s research community. The quality and impact of the work being produced across the University reflects a joint dedication to excellence in an increasingly complex and evolving research administration landscape. It has been a rewarding experience to engage with this community, and I look forward to supporting continued momentum in the year ahead. In the coming months, I am also eager to see the impact of the Novelution implementation as it replaces COEUS and enhances our ability to support researchers by providing greater flexibility and efficiency in our administrative processes.
The Office of Research & Innovation remains committed to providing consistent, transparent, and responsive support to our academic units and research teams. We look forward to continuing our collaboration throughout the year and welcome your feedback as we work together to strengthen and enhance the University’s research enterprise.
Best,
Gia Boersema
Questions? Please email Gia Boersema, Assistant Vice Provost, Sponsored Programs at gia.d.boersema@drexel.edu.
General
Celebrating and Welcoming ORI Team Members
Congratulations to Elan Mitchell-Gee, Ph.D., Director of Export Control and Research Security
Dr. Mitchell-Gee recently completed the Export Compliance Training Institute’s (ECTI) Certified Export Compliance Professional (ECoP) Export Administration Regulations exam, earning a highly regarded certification in export compliance. A valued member of the Office of Research & Innovation since 2023, she adds this credential to her extensive expertise in export control and research security. Elan holds a Bachelor of Arts in International Studies from Spelman College and a Ph.D. in International Relations from Howard University. Please join us in congratulating her on this accomplishment!
Congratulations, Sherri Reneski, on her recent promotion to IRB Analyst!
Sherri Reneski, a member of the Human Research Protections Team, was recently promoted to an IRB Analyst. Sherri joined the Office of Research & Innovation in 2022 as an IRB Coordinator with over seven years of experience managing client relationships and complex deliverables for national and international accounts. Through this background, Sherri developed both practical knowledge in regulatory compliance and a strong commitment to its role in professional practice.
In her previous roles as Account Manager and Logistics Coordinator, Sherri applied regulatory requirements by maintaining compliant documentation, monitoring certifications and deadlines, and addressing operational and compliance-related challenges.
Sherri also served as a Crisis Counselor for the Penn State and Centre County communities, having trained in short-term crisis intervention. This role required strict adherence to HIPAA privacy regulations and the American Psychological Association's ethical code of conduct in all aspects of client interaction, documentation and records management. Sherri upheld these standards while navigating complex ethical considerations to protect client confidentiality and welfare.
She earned her BA in Psychology from Penn State University.
Sherri’s colleagues appreciate her calm, kind attitude, and her dedication to providing exceptional support to Drexel’s research community. The ORI is excited for her to take on this new position!
Welcoming New ORI Team Members
Please join us in welcoming our new colleague to the following teams:
Joining the Research Compliance and Regulatory Affairs team
Dr. Sarah Archibald, Associate Vice Provost for Research Compliance & Regulatory Affairs. We’re excited to share the appointment of Dr. Sarah Archibald as the Office of Research & Innovation’s new Associate Vice Provost for Research Compliance & Regulatory Affairs!
Sarah has extensive research compliance experience, with over 20 years of leadership in higher education, overseeing complex ethics and regulatory programs across research, civil rights, and academic environments. She has coordinated cross-functional compliance initiatives, implemented enterprise-wide auditing protocols, and fostered university cultures grounded in accountability and ethical decision-making.
She is recognized for her expertise in designing and executing comprehensive compliance programs, developing institutional frameworks for risk mitigation and federal compliance, training investigators and stakeholders in ethical conduct and regulatory policy, and interfacing with federal agencies, external auditors, and governance boards, among many other accomplishments.
Most recently, Sarah served as Executive Director of the Human Research Protections Office at the University of Maryland, Baltimore, where she led strategic planning and accreditation activities for ethical human subjects research. Prior to that position, she held the roles of Director of Auditing, Monitoring, and Investigations as well as Research Integrity Officer, advising senior leadership, directing remediation efforts, and aligning policy development with evolving federal mandates.
In her new role in the Drexel University Office of Research & Innovation, Sarah will provide enterprise-wide oversight to prevent, identify, respond to, mitigate, and monitor research compliance. She will also serve as a liaison between the Office of Research & Innovation and faculty and staff on matters related to research compliance, including human research protections, animal use and care programs, export control, research security, research integrity, responsible conduct of research, research conflicts of interest, Institutional Review Boards (IRB), Institutional Animal Care and Use Committee (IACUC), Institutional Biosafety Committee (IBC), and related regulatory requirements.
Sarah will be joining us on Monday, February 9, stepping into Cassie Myers’ former role. She will play a pivotal part in the Novelution implementation and in integrating research compliance activities into a comprehensive, institution-wide research compliance program that will help expand the impact of Drexel’s research enterprise.
We look forward to welcoming Sarah to the ORI and introducing her to the full research community as she settles in. We are eager to start the new year with such an outstanding addition to our team!
Joining the Sponsored Programs team
Alexandra Sedehi, Lead Contracts Negotiator. We are thrilled to announce that Alexandra Sedehi, J.D., has joined the Office of Sponsored Programs as a Lead Contracts Negotiator.
Alexandra holds a Bachelor of Arts in History from Rutgers University and a Juris Doctor from Brooklyn Law School.
She has extensive experience in drafting and negotiating contracts, from NDAs and MOUs through CRA, SRA, and CTAs, federal procurement, and compliance, as well as grants and sponsored research administration. Additionally, she demonstrates a proven track record of managing research contracts with the federal government, non-profit, and for-profit sponsors during her fourteen years at Johns Hopkins University, her work at the Stand Up to Cancer Foundation, and, more recently, at the National Heart, Lung, and Blood Institute.
In her new role, Alexandra will support Drexel's research community by negotiating and administering contracts and agreements for sponsored projects and for funded and unfunded research activities, providing expertise and support on contractual matters.
Please join us in welcoming Alexandra to Drexel!
Joining the Core Facilities team
Nicole Bohn, Research Instrumentation Specialist. Nicole received her undergraduate degree in science, technology, and society from the University of Pennsylvania, where she got her first experiences as a scanning electron microscopy trainer. Since then she has supported a broad base of researchers on electron, scanned probe, and X-ray microscopes in shared instrumentation facilities at MIT and most recently at UPenn.
In her new role, Nicole will oversee some of the most highly utilized and impactful instruments in the MCC including the Apreo SEM and NanoCT. Along with her documented technical expertise in electron and X-ray microscopy, Nicole is known for being an adaptable, innovative instructor who focuses on the needs and outcomes of researchers with all levels of technical experience.
Nicole is an avid Phillies fan counting down to opening day. When not at a game, she enjoys crafting, cooking, reading, and gaming with her South Philly community or exploring outside the city with her partner.
Aleah DuBose, Animal Caretaker, ULAR. We’re excited to welcome Aleah DuBose to our animal caretaker team. Aleah brings a strong work ethic, great communication skills, and a genuine care for animals to her role. She’s known for being reliable, detail-oriented, and a supportive team player who takes pride in doing things the right way. Aleah is a fast learner with a positive attitude, and we’re looking foreard to have her join our animal care and husbandry team.
Dylan Herbil, Animal Caretaker, ULAR. Please join us in welcoming Dylan Herbil to the ULAR team. Dylan joins us from the University of Pennsylvania, where he gained solid hands-on experience in animal care, husbandry, and cagewash. He’s known for being reliable, hardworking, and a great team player who isn’t afraid to take initiative. Dylan is also AALAS certified and eager to keep learning and growing in his role. We’re excited to have him on board and look forward to working with him!
Rakki Taylor, Animal Caretaker, ULAR. Rakki brings with him several years of hands-on experience in animal care and cage wash operations from his time at the University of Pennsylvania, along with a strong background in training, operations, and facility support across a multi-building facility. In his new role with University Laboratory Animal Resources (ULAR), Rakki will be supporting daily animal care operations at the Queen Lane Facility, performing husbandry, and working closely with both veterinary care and laboratory staff to help maintain the high standards of animal welfare we uphold across our facilities. He is known for being reliable, team-oriented, and always ready to jump in, learn something new, and contribute wherever needed. We’re very happy to have Rakki join our team and look forward to the experience, dedication, and positive energy he brings to ULAR. Please join us in giving him a warm welcome!
Questions? Please email the Office of Research & Innovation's HR team at ori_hr@drexel.edu.
Novelution Implementation Update: Upcoming Webinar & User Acceptance Testing Opportunities
We are excited to share updates on the implementation of Novelution, which will replace COEUS as the Office of Research & Innovation’s central electronic research administration system.
After a busy fall focused on system planning and configuration for the IRB and Sponsored Programs Modules, preparations for testing and readiness activities are underway. The project team is actively validating workflows to ensure the system meets the needs of our research community.
Looking ahead, the Novelution Implementation Team will host a webinar on Tuesday, February 17, from 12–1 PM. The session will include live demonstrations, practical walkthroughs, and time for Q&A.
We are looking forward to helping you become familiarized with the new system! We are currently organizing User Acceptance Testing (UAT) and developing training materials to help ensure a smooth transition to Novelution. UAT will allow users to provide direct feedback and help us optimize resources and support. If you are interested in participating in UAT, please sign up here. Your input is instrumental in shaping a system that is driven by the needs of those it impacts most.
Novelution will offer many benefits that will improve and streamline functionality, including clearer workflows, improved task management, and more effective notifications. As always, be sure to check out the Novelution Hub for continued updates and an FAQ. The Hub will also be the future home of Novelution training materials and other important resources.
Questions? Please email the Novelution Implementation Team at novelution_info@drexel.edu.
The ORI Annual Report for AY ‘24-‘25 is Now Available!
The Drexel University Office of Research & Innovation Annual Report is now live for Academic Year 2024–2025!
This report is a testament to the dedicated work Drexel’s diverse and committed research community and the individuals that support advancing knowledge creation and application on a global scale. The report highlights milestones and achievements across our ORI departments, along with an overview of resources available to our researchers. Inside, you’ll find an introduction by Aleister Saunders, metrics on our activities, exciting research news stories, information about beneficial partnerships, memberships and initiatives, and other valuable topics that demonstrate the broad impact of the ORI and its value to the research community.
It reflects the meaningful work and deep dedication of our team, which allows Drexel to continue advancing as a world-renowned research institution. We’re proud to share our accomplishments with you and we invite you to celebrate another tremendous year of growth and impact!
Questions? Please email Becky Campbell, Senior Business Analyst, at becky.campbell@drexel.edu.
Highlighting Drexel News Research Stories: Breakthrough on Gene Therapy for Hereditary Spastic Paraplegia
We’re excited to share a recent Drexel News story that dives into impactful new research! Drexel University researchers, working with colleagues at UMass Chan Medical School, have made a promising advance toward treating Hereditary Spastic Paraplegia (HSP), a rare genetic disorder that affects movement and currently has no cure. Using a new “silence and replace” gene therapy approach, the team was able to stop, and in some cases reverse, nerve damage and movement problems in a mouse model of the disease. Led by Drexel University College of Medicine's Peter Baas, PhD, and Emanuela Piermarini, PhD, the study, published in Molecular Therapy, offers early evidence that gene therapy could one day slow or prevent disease progression in patients and highlights the impact of collaborative, patient-driven research.
Read the full story on Drexel News
Questions? Please email Greg Richter, Assistant Director, Medical, Public Health and Biomedical Engineering Communications, at gregory.d.richter@drexel.edu.
Drexel Advances Medical Research with New University City Life Sciences Facility
Drexel is taking a major step forward in medical research with a new home for the College of Medicine’s research operations at 3201 Cuthbert Street in University City. The state-of-the-art facility will bring together research teams currently based in Center City and East Falls into more than 150,000 square feet of modern lab space, designed to foster collaboration, accelerate discovery, and strengthen Drexel’s role in Philadelphia’s growing life sciences ecosystem. The move aligns Drexel’s research enterprise more closely with the University’s academic core and positions the institution for long-term impact and growth! Occupancy is anticipated following the building’s completion in 2027.
Read the full story on Drexel News
Library Support for Open Access Publishing
The Drexel Libraries currently has agreements with 10 publishers to cover author publishing charges (APCs) for Drexel corresponding authors. Publishing partners include ASC, Cambridge University Press, Elsevier, Springer Nature, and new for 2026: an agreement with Wiley. Visit the Libraries’ Open Access Publishing guide to learn how you can publish your research as Open Access for free.
Questions? Please email libsystems@drexel.edu.
New to Altmetric Explorer: Attention Digest Feature
The vendor Altmetric has announced the Beta launch of the Altmetric Attention Digest, a new AI-powered feature that generates concise summaries of key metrics, influential sources, and overall reception for individual research outputs. The Attention Digest button is available on all Altmetric Details pages -- check out this example.
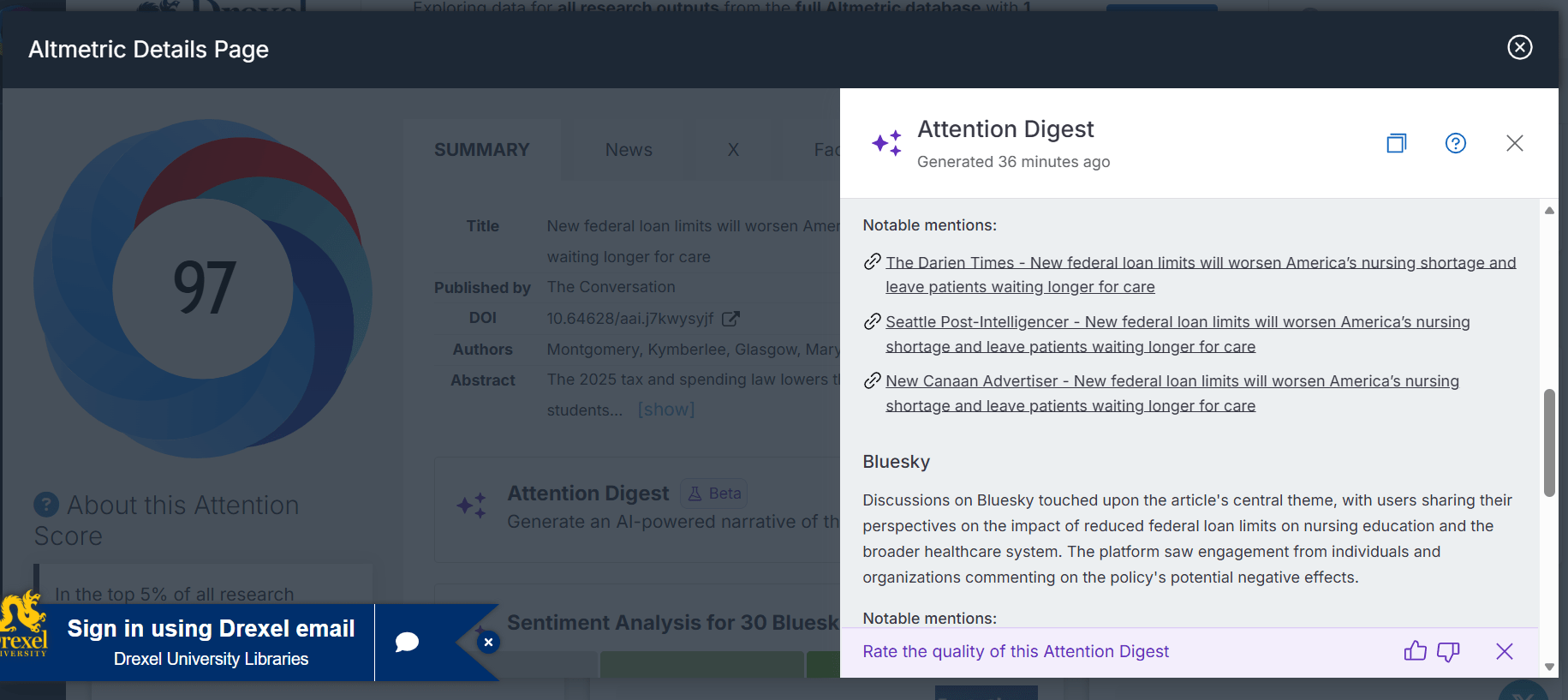
Access Altmetric Explorer and explore the new Attention Digest feature via the Drexel Libraries.
Please email libsystems@drexel.edu to share your feedback on this new feature, particularly the quality of the content is provides.
Applied Innovation
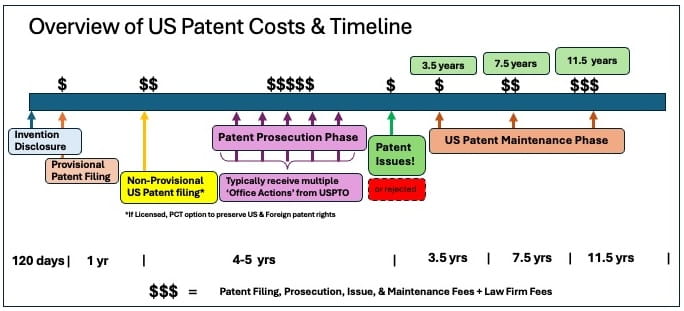
Did You Know? Patent Processes & Guidelines
When Applied Innovation, the University’s technology transfer office, receives a disclosure about a possible invention, we evaluate the innovation for patentability as well as commercialization and licensing potential before deciding on intellectual property protection. In some cases, patent protection may not be relevant, such as copyrights for software, but those technologies can still be licensed.
Drexel researchers may be surprised to know what’s involved in moving an invention through the US patent process. Some key facts & figures:
- Drexel has more than 700 issued or pending patents, US and foreign, most older and unlicensed.
- It may take up to 5-6 years of patent prosecution to get a US patent issued; however, not all patents will get issued.
- Patent filing & legal fees can cost up to $10-40k per US patent.
- Most costs occur after initial filing and costs can span over 15 years .
- Inventions resulting from Federal funding must be reported, and the US government retains rights to those patents.
- Following best practices, we focus on US protection as the world’s primary market and will pursue international filings only with a Licensee.
- We rely on Licensee revenue and patent reimbursements to help fund the protection of new inventions.
- Older patents, low technology readiness, and inventors who have left Drexel are some factors that can make technologies difficult to license.
- A patent alone is not a product. It takes more time, money, and translation for inventions that address unmet market needs to move to commercialization and create impact.
- Decisions to start, continue or abandon patents for Drexel are made by Applied Innovation.
As our team works with researchers to evaluate new disclosures, licensing managers can describe the patent process and answer questions you may have.
We manage IP assets by reviewing the age, stage, and commercial prospects of Drexel patents on an ongoing basis. Our focus is on reducing and re-balancing a large patent portfolio and investing limited resources in technologies from current Drexel researchers that have the potential for translation, licensing, commercialization, and impact.
Questions? Please email the Applied Innovation team at applied_innovation@drexel.edu.
Core Facilities
Registration for Winter Research Computing Workshops Now Open
The University Research Computing Facility (URCF) is pleased to announce our Winter 2026 workshop series, which will run from February 17-24.
The URCF, part of ORI Core Facilities, provides support for computational research at Drexel. These workshops are open to all members of the Drexel community and will be useful for anyone interested in using computation in their research, regardless of discipline. They teach programming basics, as well as how to access and use Picotte (Drexel’s high-performance computing cluster). They’re interactive, so you’ll practice these skills during the workshop using your own computer.
Register or get more information on the URCF website here
Questions? Please email James Porter, Research Computing Specialist, University Research Computing Facility, at james.jared.porter@drexel.edu.
Update on SEM, XRM Trainings at the Materials Characterization Core
The Materials Characterization Core is pleased to announce the return of trainings for the TFS Apreo 2S Scanning Electron Microscope (SEM) and the Zeiss Xradia Versa 620 X-ray Microscope (nanoCT/XRM). More information about these equipment can be found on our website.
Equipment training invites researchers to learn how to independently operate advanced characterization equipment through the thoughtful instruction and support of staff specialists. Training is best suited for researchers who intend to use equipment with relative frequency. For less frequent use, staff may be available to provide characterization support.
Due to the extended absence of a trainer for SEM and XRM, there may be a 3-4 week wait before processing new requests.
If you or a researcher in your group is interested in SEM or XRM training, please register for an iLab account then submit a training request.
Questions? Please email Nicole Bohn, Research Instrumentation Specialist for SEM and XRM, at Nicole.erika.bohn@drexel.edu.
Research Compliance & Regulatory Affairs
ORI Guidance and Procedure Update
As the Office of Research & Innovation (ORI) continues its efforts toward Drexel University’s strategic plan and CLARITY project, we are excited to introduce a new and updated SOPs and guidelines through ORI’s established workgroup processes that will continue to provide best practices, enhance collaboration, and reinforce our commitment to Drexel University and our research community:
IACUC Procedure UpdatesACU-009: Grant Congruency Check [PDF]
-
Federal regulations, including PHS Policy and the NIH Grants Policy Statement, require institutions to confirm—prior to award—that all proposed animal work has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC). This process, known as Grant Congruency Check, ensures that the research described in grant applications aligns with approved IACUC protocols.
-
Please complete this Grant Congruency Check Form [PDF] to submit a request to the IACUC Office.
-
Effective 01/01/2026
ACU-007: Investigating Animal Welfare Concerns & Noncompliance Activities – Reporting Form [DOC]
-
Drexel is committed to providing humane use, care, and treatment of research animals. In conjunction with previously released procedures for ACU-007: Investigating Animal Welfare Concerns & Noncompliant Activities, this new reporting form allows simple, direct reporting of animal welfare concerns to the IACUC office. Reports may be made anonymously.
-
Effective 01/05/2026
Questions? Email Liz Hann, Director of Animal Welfare, at iacuc@drexel.edu.
IRB/HRP Procedure Updates
HRP-030: Education and Training [PDF]
- These procedures outline the training resources and requirements, initial and ongoing, for Drexel University IRB members, IRB staff, the HRPP office, investigators, and members of the research team related to ethical concerns and regulatory requirements for the protection of human subjects.
- Effective 11/10/2025
HRP-050: Human Subjects Research Determination [PDF]
- The purpose of this procedure is to outline the process for determining whether an activity constitutes human subjects research and, if so, whether the institution is engaged in the research, in accordance with applicable federal regulations and institutional policies.
- Effective 11/10/2025
HRP-440: Trainee or Student Research and Course Projects [PDF]
- These procedures apply to student and trainee researchers when research involving human subjects is part of a course project. They describe activities that are considered human subjects research as well as those course activities which would not be considered human subjects research and the responsibilities of the course instructor to inform students about the ethical responsibilities of conducting of human subjects research.
- Effective 11/10/2025
HRP-450: International Research [PDF]
- The Drexel University Institutional Review Board (IRB) will review international research involving human participants to ensure adequate provisions are in place to protect the rights and welfare of the participants. These procedures describe the considerations and responsibilities for conducting international research projects.
- Effective 11/10/2025
Feedback or Questions? Please email Marisa Corbett, Executive Director of Research Quality Assurance, at marisa.jo.corbett@drexel.edu.
Research Security Training Update
The institution is transitioning to a standardized one-hour Research Security training module delivered through CITI. Completion of this module satisfies research security training requirements established by the National Science Foundation (NSF) and other federal funding agencies, consistent with current federal guidance.
While NSF began requiring research security training for senior/key personnel on proposals submitted on or after October 10, 2025, NIH has announced that its own research security training requirement will take effect for applications submitted on or after May 25, 2026, as outlined in NIH Notice NOT-OD-26-017.
The Office of Research & Innovation recommends that all researchers begin completing the one-hour training now in anticipation of expanding requirements.
Reminders
- If you previously completed Drexel-approved research security training, you will not need to complete the new module until your next annual renewal deadline.
- Additional CITI modules (e.g., Undue Foreign Influence, Export Controls) may be required for certain controlled, sensitive, or elevated risk projects.
Access Instructions
The required one-hour condensed Research Security training is available through CITI. To add the required Research Security module to your CITI account:
- Log in to the updated CITI Training Portal.
- Select View Courses for Drexel.
- You will see all the courses you completed for Drexel.
- Scroll to the bottom of the page and select “Add a Course.”
- Under available course options, select: Research Security.
- Then select Research Security Training (Combined Course)
This is the Condensed Research Security (1 hour) module. - The module will then appear in your course list and can be launched immediately.
- Find detailed instructions in the ORI Informational and Training Resource Library.
Completion is automatically tracked in CITI for institutional compliance purposes and meets NSF, NSPM-33, and CHIPS and Science Act research security training requirements.
Thank you for your attention to these federal compliance requirements and for your ongoing commitment to secure and responsible research practices.
Questions? Please email the Research Security team at researchsecurity@drexel.edu.
Sponsored Programs
Federal Sponsor Updates
Policy Notice: Implementation of Policy Changes to Proposal and Award Policies and Procedures Guide (PAPPG) 24-1, Supplement 2
January 22, 2026 - Although publication of the PAPPG has been deferred, NSF will continue to issue financial assistance policies to support administrative priorities, implement statutory requirements, document current practices, and address other time-sensitive matters. During this interim period, NSF will communicate policy updates through supplemental Policy Notices.
NOT-OD-26-026: NIH Requires Use of the eRA Prior Approval Module for the Submission of ALL Prior Approval Requests
January 21, 2026 - NIH will now require that all prior approval requests for grant and cooperative agreement awards be submitted electronically through the eRA Commons Prior Approval Module, effective 30 days from the date of the notice. This change streamlines submission and review, requires requests to be initiated by the organization’s Signing Official, and will be incorporated into an upcoming update of the NIH Grants Policy Statement.
NOT-OD-26-018: NIH’s Implementation of Common Forms for Biographical Sketch and Current and Pending (Other) Support for Due Dates on or after January 25, 2026
December 2, 2025 - To promote collaboration across federal research agencies, NIH is adopting the Common Forms for Biographical Sketch and Current and Pending (Other) Support, consistent with the OSTP memorandum on the use of common disclosure forms. This Guide Notice outlines requirements for the Common Forms, the NIH Biographical Sketch Supplement, and related instructions for applications and RPPRs due on or after January 25, 2026. Institutions are also reminded to maintain internal disclosure controls, including policies, procedures, and required training for senior/key personnel. Forms must be completed in SciENcv and linked to an ORCID iD, with certification by all senior/key personnel. Forms include declarations regarding participation in foreign talent recruitment programs.
NIH 26-019: Admin Burden Reduction, Removal of LOI Requirement from Some Applications
December 3, 2025 - Letters of Intent (LOIs) are no longer required or accepted. Prior approval requirement for unsolicited applications ≥ $500,000 in direct costs is removed.
NIH 26-017: Research Security Training Requirement for NIH
December 2, 2025 - New research‑security training requirement: Senior/key personnel on NIH grant applications must complete mandated Research Security Training (RST). Certification required at submission. Each covered individual and the submitting institution must certify RST completion.
NIH 26-005: Interim Guidance on Reopening Extramural Activities
November 14, 2025 - NIH is restarting its extramural (grant & contract) operations after the federal government shutdown — including rescheduling all grant application deadlines. NIH warns that support help-desk services may be slower as staff catch up with backlog and will send out confirmations and communications soon.
Implementing a Unified NIH Funding Strategy to Guide Consistent and Clearer Award Decisions
November 21, 2025 - Core tenets emphasize aligning with NIH’s mission, prioritizing scientific merit, supporting a broad research portfolio, considering investigator career stage and geographic distribution, and grounding decisions in ICO budget realities. NIH will centralize funding policy information to improve transparency and accountability across all ICOs. ICOs will eliminate paylines and instead use full peer review critiques in context with strategic priorities and budgets to reduce confusion.
NSF 149: Updates to NSF 149 Research Security Requirements
November 24, 2025 - Mandatory research-security training: All senior/key personnel must complete approved training within 12 months before NSF proposal submission. Stronger disclosure requirements: Institutions must maintain and provide documentation of foreign affiliations, contracts, and support when requested.
Visit the Sponsor Updates Webpage
Questions? Please email Gia Boersema, Assistant Vice Provost, Sponsored Programs, at gia.d.boersema@drexel.edu.
Office of Sponsored Programs Lunch & Learn “How to” Series
The Office of Sponsored Programs happily announces the return of its Lunch & Learn “How to” Series. This third season will be condensed into four episodes, with the last episode held in May.
Here's a look at the first two episodes:
Episode 1: Closeout
February 10, 2026 at 12 - 1 PM
Join us to learn about the three sections of the NIH award closeout process. During this session, we’ll walk through closeout requirements and provide an overview of the Final Financial Report (FFR), Final Research Project Progress Report (FRPPR), and Final Invention Statement (FIS), helping ensure a smooth and timely closeout.
Episode 2: Grants vs. Contracts: Who is Responsible for What?
March 10, 2026 at 12 - 1 PM
Register for each episode here:
- Season 3, Episode 1: "Closeout”
- Season 3, Episode 2: "Grants vs. Contracts: Who is Responsible for What?"
- Season 3, Episode 3: TBD
- Season 3, Episode 4: TBD
Questions? Please email Carissa Lynn Miller, Research Compliance Coordinator, at carissa.miller@drexel.edu.
Global Work Requirements for Independent Contractors and Beyond
Please be advised that all paid and unpaid work performed on behalf of Drexel University outside the United States must be reviewed and approved in advance by the Global Work Review Committee. Departments requesting individuals, such as non-employee associates, independent contractors, or collaborators, to work abroad are required to begin the process by completing the Drexel University Intake Form for Paid and Unpaid Work Based Outside the United States.
This review helps identify potential legal, tax, safety, data security, and compliance risks before any work or system access begins. The form applies to a wide range of global work arrangements, including research assignments, short-term expert engagements, and long-term international relocations. Requests are evaluated to determine the appropriate global workforce solution.
Global Work Committee approval should accompany a contract request.
Learn More
Questions? Email the Global Work Review Committee at globalhr@drexel.edu.
New Resource Sheet Helps Researchers Understand Residential Segregation
The Urban Health Collaborative’s (UHC) Research & Data Core has released a new resource sheet to help students and researchers better understand how residential segregation is measured in public health research. The guide outlines several commonly used quantitative measures, including the Dissimilarity Index, Gini Index, Index of Concentration at the Extremes (ICE), and the 𝑮𝒊∗ (Getis-Ord Gi-star) statistic, and provides formulas, key references, and examples of how each has been applied in empirical studies.
Understanding residential segregation is critical for examining the ongoing health impacts of structural racism and discriminatory housing policies such as redlining, which continue to shape outcomes related to cancer, asthma, hypertension, preterm birth, cardiovascular health, and more. By clarifying the strengths and applications of different segregation measures, this resource aims to support more consistent and informed use of these tools in public health research.
Questions? Please email Will Becker, Marketing & Communications Specialist, Urban Health Collaborative, at will.becker@drexel.edu.
Drexel Researchers Receive New Awards!
We’re proud to share that during Q2 of FY 2026, Drexel researchers have secured 57 new awards, totaling $8.1 million in funding. These awards reflect a diverse range of support, including:
- 36% from federal sponsors
- 29% from private foundations
- 11% from non-profit organizations
Congratulations to the investigators below on the following projects, which reflect a selection of the largest awards granted this quarter:
- Crucial Spinal Circuit Changes that Mediate Locomotor Benefits and Deficits Following Combined Therapies After Spinal Cord Injury
Dr. Kimberly Dougherty – Neurobiology and Anatomy, College of Medicine
Dr. Simon Giszter – Neurobiology and Anatomy, College of Medicine
NIH National Institute of Neurological Disorders and Stroke
Drs. Kimberly Dougherty and Simon Giszter have received five additional years of funding from the NIH for their multi-principal investigator R01 award, continuing their collaborative work to identify strategies to both improve locomotor function and limit motor deficits following spinal cord injury. This is a renewal grant that builds upon the team’s findings from the prior funding period that showed that viral delivery of brain derived neurotrophic factor into the spinal cord improved stepping abilities in rodents with complete spinal cord injuries, but the improvements were often severely limited by the concomitant development of overactive reflexes. When the viral neurotrophic factor treatment was combined with daily spinal stimulation, the overactive reflexes were mitigated, and stepping ability was vastly improved. This project assesses behavioral and electrophysiological outcomes following these combination treatments in order to reveal the changes in below-injury innervations by sensory neurons, activity of spinal neurons, network connections between neurons, and patterns and timing of muscle activations, focusing on their differences between animals that recover stepping and those that develop harmful reflexes that compromise stepping. This knowledge may reveal biological markers for pathology and the likelihood of beneficial response to interacting therapies. The project is expected to uncover new targets for treatments to improve walking after spinal cord injury.
- Boosting Vaccine Durability for the Elderly Through ADA‑1 Adjuvant Innovation
Dr. Michele A. Kutzler, PhD – Medicine and Microbiology & Immunology, College of Medicine
Merck & Co., Inc
A new Merck Investigator Studies Program–funded project totaling $463,000 led by Dr. Michele Kutzler in the College of Medicine aims to transform vaccine efficacy in older adults by targeting one of the most urgent challenges in public health: age‑related immune decline. As people age, immunosenescence reduces the quality of T and B cell responses, and impairs germinal center reactions, a key process that occurs inside specialized structures of lymph nodes and the spleen called germinal centers (GCs). It is one of the most important steps in generating high‑quality, long‑lasting antibody responses after infection or vaccination. Thus, in the aged, weakened antibody durability leads to poorer outcomes following infection or vaccination. This study evaluates Adenosine Deaminase‑1 (ADA‑1) as a molecular adjuvant to restore these critical immune pathways in the elderly.
Using Clostridioides difficile infection (CDI) as a model system, and a patented nucleic acid-based vaccine developed by Dr. Kutzler (US Patent 9446112), the team will test whether ADA‑1 can enhance mucosal and systemic immunity to nucleic acid vaccines targeting the major C. difficile toxins (TcdA and TcdB). CDI is a particularly relevant model: the grant highlights CDI as a major source of morbidity, mortality, and healthcare cost in the U.S., exceeding $1.1 billion annually and showing a 23% annual increase in cases in hospitals and long‑term‑care facilities since 2000. Importantly, over 90% of CDI‑associated deaths occur in individuals older than 85, making it one of the top infection‑related killers of older Americans. The elderly also experience markedly higher recurrence rates and more severe disease, largely due to impaired antibody responses and diminished T follicular helper (TFH) cell function.
In collaboration with Dr. Elias Haddad and Dr. Chris Sell, the research team has generated preliminary data in aged mice demonstrating that ADA‑1 can restore TFH activity, boost anti‑toxin antibody responses, and improve germinal center reactions, effectively converting an age‑impaired response into one resembling that of young adults, in research that was carried out by graduate student Emily Konopka (PhD student in the Department of Microbiology and Immunology). Drs. Kutzler and Haddad have patented ADA-1 as an immune modulator for vaccines and cancer immunotherapies (US Patent 12064481), and this project will expand those findings to evaluate protection from primary CDI and recurrence.
Merck’s support is pivotal: ADA‑1 represents a next‑generation precision adjuvant that could improve durability, breadth, and mucosal immunity across vaccine platforms—not only for CDI, but potentially for influenza, SARS‑CoV‑2, RSV, and other pathogens that disproportionately threaten the elderly. This research marks an exciting step toward vaccines that remain effective across the human lifespan.
-
Accelerated Pathways to Impact: A Competency-Based, Three-Year MD Program at Drexel University College of Medicine
Dr. Leon McCrea – College of Medicine
Independence Blue Cross
Drexel University College of Medicine (DUCOM) Office of Educational Affairs (OEA) has received a $425,000 award from Independence Blue Cross to support a planning-year initiative led by Dr. Leon McCrea, Vice Dean of Educational Affairs, to design a three-year, competency-based MD pathway. The project focuses on developing an accelerated medical education model that addresses shortages in primary care specialties. This initiative positions DUCOM to pilot an innovative, scalable model of medical education that aligns academic excellence with workforce and health equity priorities.
-
Community-Driven Air Quality, Heat, and Humidity Mitigation in Environmental Justice Neighborhoods of Philadelphia
Dr. Michael Waring – Civil, Architectural and Environmental Engineering, College of Engineering
William Penn Foundation
Drexel University is conducting a community-centered project to reduce exposure to indoor air pollution, extreme heat, and high humidity in environmental justice neighborhoods in Philadelphia. Led by Dr. Michael Waring, the project integrates environmental sensing, data-driven modeling, and practical household interventions with strong partnerships among local community-based organizations.
The project combines indoor and outdoor environmental monitoring with predictive modeling and mapping tools to better understand how environmental conditions affect residents inside their homes. These data support real-time, home-specific guidance delivered directly to residents through accessible communication channels, enabling timely protective actions during periods of elevated risk.
Community engagement is central to the work. Local organizations guide outreach, implementation, and interpretation of results, while residents and youth participate through workshops, participatory science, and leadership opportunities that build environmental literacy and local capacity. By pairing low-cost mitigation strategies with training and ongoing guidance, the project aims to reduce indoor environmental burdens while creating a replicable, community-driven framework for improving environmental health and resilience.
Seeking funding?
Search for funding opportunities using Pivot and Funding Institutional
Let’s keep the momentum going!
Questions? Please email Rose Ann DiMaria-Ghalili, Interim Associate Vice Provost for Research & Innovation, at rose.a.dimaria-ghalili@drexel.edu.
Faculty Highlights: Recent Awards and Grants
Drexel News recently spotlighted investigators who have exciting, new sponsored research happening across the University. The ORI is thrilled to feature these researchers and their recent accomplishments!
- Margaret Finley, PhD, and Laura Baehr, DPT, PhD, from the College of Nursing and Health Professions, received a $3.2 million Spinal Cord Injury (SCI) Research Program Clinical Trial Award from the U.S. Department of Defense. This randomized controlled trial will evaluate the effectiveness of tele-exercise to promote empowered movement in individuals with SCI through an online group exercise program, compared to a self-guided video library, to improve physical activity, exercise beliefs, quality of life, pain, sleep and wellbeing.
- Rikki Patton, PhD, and several co-investigators in the College of Nursing and Health Professions, received $2.4 million from Health Resources & Services Administration to launch the Strengthening Healthcare through Interprofessional Networks and Education (SHINE) Program. The SHINE program is a training initiative designed to strengthen the behavioral health workforce in the Philadelphia region through interprofessional collaboration and advanced training in youth suicide prevention and crisis management.
- Adaobi Anakwe, PhD, in the Dana and David Dornsife School of Public Health, was awarded a Changemakers in Family Planning training grant of $83,919 by the Society of Family Planning. This training grant supports the development of a body of research that illuminates contraceptive decision-making processes among Black young adult men (and fathers) and enhances community-led strategies that increase their uptake of family planning interventions with attention to the systemic/structural facilitators and barriers to uptake.
- A U.S. Department of Agriculture grant project led by Vera Lee, EdD, in the School of Education, introduced agriculture and food science to 22 high school teachers and guidance counselors. They learned about the many different career options in the food industry and developed lesson plans and resources that they can apply in their schools.
Read the full story on Drexel News
Questions? Please email Becky Campbell, Senior Business Analyst, at becky.campbell@drexel.edu.
Drexel Researchers Awarded AWIS Bridge Grants
Four Drexel University researchers have been selected to receive Association for Women in Science (AWIS) Bridge Grants, which provide critical funding support to researchers impacted by recent federal funding cuts. Each $5,000 award is intended to help recipients continue their research momentum and support trainees during this transitional period.
The Drexel recipients are:
- Kristal Lyn Brown, PhD, MSPH – Assistant Professor, CNHP - Creative Arts Therapy
- Jane Ellen Clougherty, ScD – Professor, SPH-Environmental & Occupation Health
- Ahmad Raeisi Najafi, PhD – Associate Professor, Mechanical Engineering & Mechanics
- Gail Rosen, PhD – Professor, Electrical & Computer Engineering
The AWIS Bridge Grants are supported by the Every Page Foundation and aim to help early-career scientists reach their next research milestone and sustain progress during a challenging funding landscape.
Congratulations, researchers!
Learn More
NASA Earth Science Mission Partnerships Calls
NASA’s Earth Science Division has released two Announcements for Partnership Proposals (AFPPs) inviting organizations to collaborate on the operations and data collection of Earth Science satellite missions. These opportunities aim to maximize the use of NASA resources, support innovative mission operations, and advance the commercial remote sensing industry.
The current partnership opportunities focus on the Cyclone Global Navigation Satellite System (CYGNSS) and the Orbiting Carbon Observatory-2 (OCO-2), with proposals due February 12, 2026. While no NASA funding will be provided, partnerships may be established through Non-Reimbursable or Reimbursable Space Act Agreements, and proposers may request specific forms of NASA assistance.
Interested parties are encouraged to monitor NSPIRES for updates, including potential virtual information sessions.
Questions? Email Beth Weinstein, Deputy Associate Director for Flight, at hq-esdpartnerships@mail.nasa.gov.
Did You Know?
"Did You Know?" is a recurring newsletter feature that shares helpful tips, tools, and guidance from Office of Research & Innovation teams to support the Drexel research community. An archive of previous "Did You Know?" sections can be found under the Resources section of the ORI website.
OSP’s Sponsor Updates Webpage
Did you find the above federal sponsor updates helpful? Good news—the Office of Sponsored Programs maintains a dedicated webpage that archives the latest sponsor updates in one convenient place!
On this page, you’ll find regularly updated news and guidance from key sponsors such as NIH, NSF, and NASA, organized by month for easy reference. It’s a great resource for staying current on policy changes, important guidance, and other updates that may impact proposal development and award management.
Be sure to bookmark the page and check back often for the latest information!
Visit the Sponsor Updates Webpage
Questions? Please email Gia Boersema, Assistant Vice Provost, Sponsored Programs, at gia.d.boersema@drexel.edu.
Research Quality Assurance Program: Good Documentation Practices
Good documentation practices help protect participants, researchers, and the credibility of the research records. Regulators and sponsors rely on documentation to confirm that study activities were conducted as approved and that data are reliable. In research, if it isn’t documented, it didn’t happen.
Helpful Tips & Best Practices:
- Document in real time or as close to the activity as possible to ensure accuracy.
- Ensure all entries are legible, dated, and attributable (who did what and when).
- Use consistent formats and approved templates whenever possible.
- Correct errors properly: single line through the error, initial, date, and explain if needed—never erase or obscure original entries.
- Avoid copying and pasting without verification; confirm that repeated entries are still accurate.
- Ensure electronic records have audit trails enabled and are access-controlled.
- Remember that notes should be objective and factual, not speculative.
- Retain documentation according to regulatory and sponsor retention requirements.
Best practice reminder: Documentation should allow an independent reviewer to reconstruct the study conduct without additional explanation.
Don’t forget to check out our Clinical Research Guidelines and Tools webpage for guidelines on good documentation practices in research and more!
Questions? Please email Marisa Corbett, Executive Director of Research Quality Assurance, at marisa.jo.corbett@drexel.edu.
Other Research Training, Education, and Meeting Opportunities
Have an event you'd like to share with the Drexel research community? Email Becky Campbell, Senior Business Analyst, at becky.campbell@drexel.edu.
Register for the Urban Health Summer Institute
The Drexel Urban Health Collaborative at the Dornsife School of Public Health is hosting its annual Urban Health Summer Institute this year from June 22-26, 2026!
The Summer Institute offers short skills-based and substantive courses for practitioners, researchers, and students of all levels interested in improving health in cities.
Some courses are in-person on our Philadelphia campus, while others are online, taught with live instruction by distinguished faculty members with broad urban and global health research portfolios.
Registration is open now! Drexel students receive a 50% discount with the code DREXELHALF.
Questions? Please email Will Becker, Marketing & Communications Specialist, Urban Health Collaborative, at will.becker@drexel.edu.
Medicine and Science Leadership Summit
Beyond the Bench: Partnerships and Policy Shaping the Future of Translational Medicine
This year’s Summit convenes leaders from basic science, clinical care, industry, and policy to explore how biomedical discoveries are translated into real-world impact. By highlighting the translational pathway alongside the collaborative and policy environments that shape it, the Summit frames translational medicine as a dynamic ecosystem—one that depends on scientific innovation, cross-sector partnerships, and informed leadership to advance equity, support careers in academic medicine, and transform discovery into lasting improvements in health.
The Summit is sponsored by the Women in Medicine and Science Committee (WiMSC) and is open to all members of the university community. We welcome researchers at all levels who are interested in building collaborations and engaging in interdisciplinary dialogue. The program will feature three expert panels offering diverse perspectives on translational science and collaborative leadership. More details coming soon!
Questions? Please email Dr. Hwyda Arafat, Professor, Department of Medicine, at hwyda.arafat@drexel.edu or Dr. Stephanie Matt, Research Assistant Professor, Pharmacology Control, at stephanie.m.matt@drexel.edu.
LabArchives Love Data Week 2026
Celebrating Data, Improving Data Management, and Increasing Productivity through the LabArchives Research Platform
The Office of Research & Innovation utilizes electronic research notebooks (ERNs) to help researchers manage the results of research efforts, record and document research processes and procedures, and manage digital research data in ways that increase reproducibility, efficiency, collaboration, searchability, and security. Drexel University has purchased an enterprise license for LabArchives ERN services for use by its faculty, researchers, staff, graduate students, and undergraduate students in performing or learning research activities.
Love Data Week is an international celebration of data that aims to promote good data practices, while building and engaging a community around topics related to research data management, sharing, preservation, and reuse. Join LabArchives for their weeklong series of events dedicated to helping you improve the organization of your data while taking steps towards better overall data management through LabArchives Research ELN, Inventory, and Scheduler. Pick and choose the sessions that interest you or attend all the sessions for a comprehensive look at LabArchives and how you can improve your research data management regimen.
Questions? Contact the LabArchives support team at support@labarchives.com.
Stay Connected with the Office of Research & Innovation!
Ways to Engage with the ORI
- Quarterly Newsletter Archive: Read past issues of our newsletter for key announcements and updates
- Novelution Hub SharePoint: Engage with the COEUS Replacement Project via our new Novelution Hub
- ORI Training Library SharePoint: Access training resources and webinar recordings
- Email Announcements: View an archive of important ORI updates
- Upcoming Events: Explore a comprehensive list of research-related events
- LinkedIn: Follow us for the latest news about Drexel research
Questions? Please email Becky Campbell, Senior Business Analyst, at becky.campbell@drexel.edu.