Office of Research & Innovation Newsletter - Summer 2024

In this issue:
Introduction from Sarah Saxton, Assistant Vice Provost, Sponsored Programs
General
- Welcoming New ORI Team Members
- NEW! Open Access Publishing Agreement with American Chemical Society (ACS)
- Learn about the Metadata Research Center's April Workshop on Navigating Metadata and Ontologies
Applied Innovation
- Drexel University Innovation Fund Opens Applications for Third Cohort
- Advancing Cancer Research Through Innovation: Dr. Emily Esquea's Journey From Lab To Real-world Impact - Stories Of Impact Series
- Dr. Greg Schwenk awarded a 2024 Activate Fellowship
- Have a Tech to Disclose?
Core Facilities
- What's Inside? Come Check Out Drexel's New X-ray Microscope!
- Cell Imaging Center Welcomes BD FACSymphony A1 Flow Cytometer
Research Compliance
- ORI Regulatory Affairs and Research Compliance Training Update
- ORI Guidance and Procedure Update
- Institutional Biosafety Committee Updates
- White House Releases Final Guidelines for Implementing NSPM-33
Research Development
Sponsored Programs
- NSF Updates
- NIH Updates
- NASA Updates
- OMB Guidance for Federal Financial Assistance Update
- NSF Spring 2024 Grants Conference
- Updated FAQs for Linked Fund Numbers on Collaborative Research Awards
- Updated Facilities and Administrative (F&A) Rates for Sponsored Programs
- Fiscal Year 2025 Approved Fringe Rates for Sponsored Projects
- Subawards and Contracts Update
Did You Know?
Introduction from Sarah Saxton, Assistant Vice Provost, Sponsored Programs
Dear Colleagues,
This summer has been a pivotal moment in Drexel's history with the merger of Salus University. We would like to extend a warm welcome to our colleagues from Salus as they become part of our research community during this transition period. I am also excited to announce that Alice Reuther has joined our Office of Sponsored Programs as the Director, Contracts and Subawards. In addition to changes across campus, key federal agencies have been reviewing current policies and guidelines to implement several changes over the next several months.
NSF recently updated the Proposal & Award Policies & Procedures Guide (PAPPG) (NSF 24-1) for proposals submitted or due on or after May 20, 2024. Key changes are in response to an increased review of malign foreign talent recruitment programs due to the CHIPS and Science Act of 2022 requirements and establishing consistency in formatting of biographical sketches and current and pending (other) support documentation. In addition, the mentoring plan requirement is expanding to include graduate students in order to meet the requirements of the revised Section 7008(a) of the America COMPETES Act of 2022.
Moreover, NIH is also preparing major changes for proposals with due dates on or after January 25, 2025. Several of the changes are to minimize administrative burden and to provide more equity in the review process. NIH fellowship and NRSA training grant applications will have the most changes forthcoming. In addition, NIH will be implementing new applications forms, FORMS-I, and adopt the Biographical Sketch and Current and Pending (Other) Support Common Forms. For RPPRs submitted on or after October 1, 2024, additional questions will be added to the reports to align with the NIH Final Policy on Data Management and Sharing. NIH is providing several webinars and recordings as well as websites devoted to specific changes.
NASA is also implementing the NSTC Common Forms for biosketches and current and pending (other) support. The agency has noted they will be making minor changes to the forms and guidance will be provided in the near future. In addition, to comply with the CHIPS and Science Act of 2022, NASA is proposing to provide a clear agency definition of senior/key personnel for the certification requirements that senior/key personnel have to certify they were not part of a malign foreign talent recruitment program.
The federal government's Office of Management and Budget (OMB) has made its first major changes to the OMB Guidance for Federal Financial Assistance, including Uniform Guidance, since it was implemented in 2013. Changes will be applied October 1, 2024; however, some changes will not go into effect until an institution has a new rate proposal or an impact statement is submitted with a rate extension request. Some important changes include increasing the equipment-related threshold from $5,000 to $10,000; increasing the de minimis indirect cost rate from 10% to 15%; and revising the definition of modified total direct costs to increase the threshold for up to $50,000 (previously $25,000) of each subaward.
With many changes occurring through Winter 2025, ORI will be providing guidance and training in the academic year along with providing links from various federal agencies. Stay tuned for multiple opportunities.
Hope everyone enjoys the rest of the summer!
Questions? Email Sarah Saxton, Assistant Vice Provost, Sponsored Programs, at arah.m.saxton@drexel.edu.
General
Welcoming New ORI Team Members
Please join in welcoming our new colleagues to the following teams:
Joining the Research Compliance & Regulatory Affairs team:
Jose Cartagena, Sr. Specialist of Export Control and COI. Jose is joining the ORI from the Center of International Trade & Security as an Energy Scholar. He holds a Bachelor degree in International Relations from Penn State and recently completed his Master's degree in International Policy at the University of Georgia. We are beyond excited to welcome him to Drexel and ORI!
Joining the Sponsored Programs team:
Alice Reuther, Director, Contracts and Subawards. Please welcome Alice, who will be responsible for the oversight of both the Contracts and Subawards Team. She will be working on process improvement, guidelines, procedures, and streamlining the work between the teams. Prior to coming to Drexel, Alice worked in research administration at the University of Virginia and Columbia University as well as HIAS, an international NGO. In addition, she has actively participated in the Federal Demonstration Partnership and is a former co-chair of the Subawards Subcommittee. Beyond her experience with subawards and contracting, she brings financial experience in post-award research accounting, internal auditing, and internal controls.
Questions? Email hire_ori_aj@drexel.edu.
NEW! Open Access Publishing Agreement with American Chemical Society (ACS)
Drexel authors may now publish open access in all ACS journals with full financial support, thanks to an agreement made possible through the Drexel Libraries' PALCI consortium membership. That means Drexel authors do not have to pay article publishing charges (APC) when publishing in ACS journals, including all ACS hybrid and transformative journals, the ACS Au collection, JACS Au, ACS Central Science, and ACS Omega. The agreement also includes full online access to ACS journals through the end of 2025.
Visit the Drexel Libraries' open access resource guide for more information Drexel's OA publishing agreements and subscribe to the Libraries' newsletter to stay up-to-date on the latest news and events.
Questions? Email Stacy Stanislaw, Director of Communications, Drexel University Libraries, at stacy.v.stanislaw@drexel.edu.
Learn about the Metadata Research Center's April Workshop on Navigating Metadata and Ontologies
Exciting times at Drexel University! In April, the Metadata Research Center hosted the NSF-Harnessing the Data Revolution (HDR) AI-Ready Data: Navigating the Dynamic Frontier of Metadata and Ontologies workshop, and it was a huge success.
With 51 experts from NSF-HDR institutes, various NSF initiatives, industry, and three U.S. national labs gathered, they dove deep into the future of AI-ready data frameworks.
Over two dynamic days, participants shared case studies, innovative methods, and strategic goals for integrating metadata and ontologies into AI workflows and informing AI-driven operations. Breakout groups sparked insightful discussions on approaches, needs, and opportunities.
Several of their key takeaways include:
- Incorporating metadata and ontologies is crucial for accelerating knowledge discovery and supporting longitudinal science.
- AI methods, including generative AI, can leverage metadata and ontologies to enhance and validate AI-ready data.
- FAIR (Findable, Accessible, Interoperable, and Reusable) data is vital for AI readiness, although not all FAIR data is AI-ready.
- Use cases help determine the level of AI readiness necessary for data.
- Metadata and ontology-informed AI techniques developed for specific domains are applicable across various fields.
- Future focus is needed on the AI-ready data spectrum, including DevOps training and AI readiness levels.
The event also included a poster session for the College of Computing and Informatics and Drexel community, which included crucial topics such as “Tying Large Language Model Data to Human Curated Knowledge Graphs,” “Relational Database Management Systems for Metadata Management and Related Machine Learning Applications,” and “FIDLAR: Forecast-Informed Deep Learning Approaches for Flood Prediction & Mitigation.”
This workshop, part of NSF-HDR-ID4: Institute of Data Driven Dynamical Design, showcased the power of collaboration and the bright future of AI-ready data. Special shoutout to Jane Greenberg, PhD, Director, Metadata Research Center, who organized the event.
Let's keep pushing the boundaries!




Learn more about the Metadata Research Center's Workshop
Questions? Email Jane Greenberg, PhD, Director, Metadata Research Center, jane.greenberg@drexel.edu.
Applied Innovation
Drexel University Innovation Fund Opens Applications for Third Cohort
Applications are now open for the Drexel University Innovation Fund, Fall 2024 Cohort. The Drexel University Innovation Fund (DUIF) makes $150,000 early-stage investments in Drexel-affiliated startups. This includes startups founded by students within a year of graduation, alumni within three years of graduation, postgraduate students within a year of graduation, and faculty members commercializing research results. Applications close August 6. Apply today.
More information on the Drexel University Innovation Fund
Questions? Email Innovationfund@drexel.edu.
Advancing Cancer Research Through Innovation: Dr. Emily Esquea's Journey From Lab To Real-world Impact - Stories Of Impact Series
As the second installment of the Stories of Impact series, Drexel Applied Innovation spoke with Emily Esquea, Drexel University College of Medicine double Drexel alumni, with a MS and PhD in Molecular and Cellular Biology and Genetics. She is the first recipient of Drexel Applied Innovation's inaugural Excellence in Expanding the Impact of Research Award at the Graduate Student Excellence Day 2024, nominated by faculty mentor, Dr. Mauricio Reginato, Professor and Chair and Director of the Graduate Program in Cancer Biology and Program Director, Translational and Cellular Oncology Program at the Sidney Kimmel Cancer Center (SKCC) Research Consortium at Thomas Jefferson University.
Read the full article for excerpts from Emily's nomination for the Excellence in Expanding the Impact of Research award, written by Dr. Reginato, and an interview with Emily on her work's impact.
Read the interview with Dr. emily Esquea
Questions? Email Lillian Rukenstein, Manager, Entrepreneurial Community, Drexel Applied Innovation, at lillian.rukenstein@drexel.edu.
Dr. Greg Schwenk awarded a 2024 Activate Fellowship
Exciting news! Greg Schwenk, a postdoc at Drexel's College of Engineering, has been awarded a 2024 Activate Fellowship, chosen from over 1,000 applicants! As CEO of 1DNano, he'll commercialize groundbreaking nanostructures with Distinguished Professor Michel Barsoum as Technical Advisor. The fellowship provides $500K+ in funding and comprehensive support to turn scientific breakthroughs into high-impact businesses.
“The Office of Research & Innovation is proud to support Dr. Schwenk and Dr. Barsoum on their exciting journey to commercialize a new material discovered at Drexel,” says Aleister Saunders, PhD, Executive Vice Provost for Research and Innovation. “This is a great example of our researchers expanding the impact of research, one of our six strategic imperatives in the Drexel 2030 Strategic Plan” says Saunders. We are incredibly proud of this achievement!
Read More About Greg Schwenk's Fellowship Award
Questions? Email Shintaro Kaido, Vice Provost for Innovation and Executive Director, Drexel Applied Innovation, at shintaro.kaido@drexel.edu.
Have a Tech to Disclose?
When Drexel innovators find something new and useful has been developed, or unusual research results have been obtained, they can take the first step in moving an innovation forward by disclosing it here for review and guidance from the Applied Innovation team. We are a dedicated partner to help generate impact from Drexel research, offering strategic intellectual property (IP) protection, entrepreneurial advancement resources and industry engagement support to faculty, postdocs, and graduate students.
Among other potential benefits, disclosing an invention (or potential invention) can help researchers get more grants (agencies like NSF and NIH value the extension of existing IP in several programs) and increase opportunities for industry collaboration.
If you're not sure if you have an invention, contact us!
Questions? Email applied_innovation@drexel.edu.
Core Facilities
What's Inside? Come Check Out Drexel's New X-ray Microscope!
Have you heard about Research Core Facilities at Drexel University? They promote discovery, creativity, and impact by providing access to advanced instrumentation and services, expertise on experimental design and data analysis, and technical training to Drexel students, researchers, faculty and external academic and industry partners. Drexel cores host some of the most cutting-edge instrumental and technical capabilities on campus and are hubs of high-impact research, innovation, collaboration and learning. They're excited to share that their recently acquired X-ray microscope, Zeiss Xradia 620 Versa nCT, is up and running. Below is a sample result acquired by graduate student Bita Soltan Mohammadlou! If you are interested in using this instrument please visit the Core Facilities website or contact Kate Vanderburgh.
Image of the Month: Drexel Zeiss Xradia 620 Versa nCT,1 MXene Coated Textile
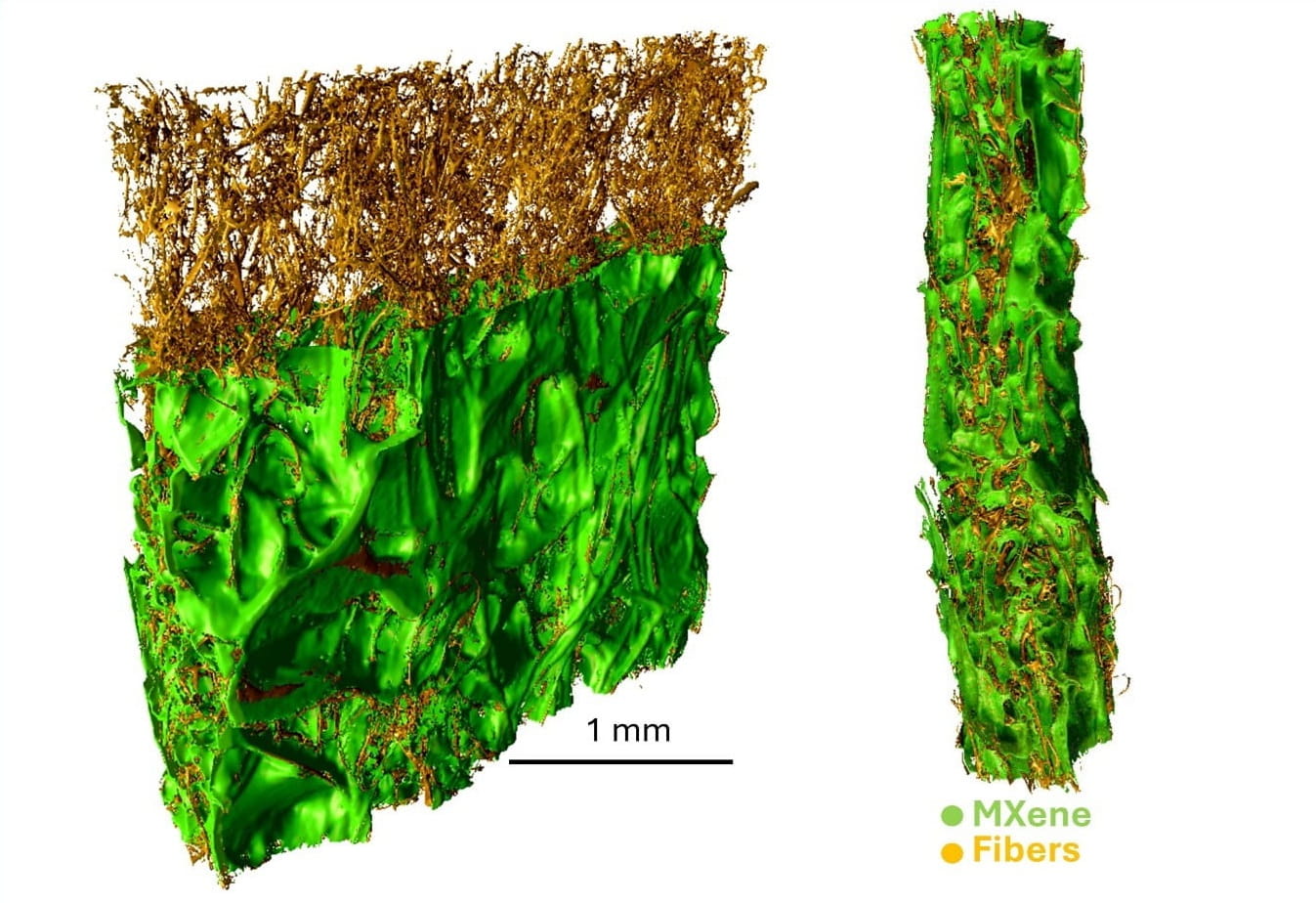
MXene enhances textiles by integrating conductivity, flexibility, and electromagnetic interference shielding, enabling the development of advanced smart textiles for applications in wearable electronics, electromagnetic interference shielding, energy storage and protective clothing.2, 3 Computerized Tomography (CT) images provide clear insights into the distribution of MXene around fibers. The primary goal is to investigate the absorption and distribution of MXene within textiles using various coating techniques such as deep coating and printing.
1 NSF MRI Award Number 2216175.
2 A. Levitt, J. Zhang, G. Dion, Y. Gogotsi, J.M. Razal, MXene-Based Fibers, Yarns, and Fabrics for Wearable Energy Storage Devices. Adv. Funct. Mater. 2020, 30, 2000739.
3 A. Inman, T. Hryhorchuk, L. Bi, R.J. Wang, B. Greenspan, T. Tabb, ... & Gogotsi, Y, Wearable energy storage with MXene textile supercapacitors for real world use. J. Mater. Chem. A, 2023, 11(7), 3514-3523.
Questions? Email Kate Vanderburgh, Research Instrumentation Specialist and Manager, SEM, Sample Prep in the Materials Characterization Core, at kate.vanderburgh@drexel.edu.
Cell Imaging Center Welcomes BD FACSymphony A1 Flow Cytometer
Cell Imaging Center's new flow cytometer BD FACSymphony A1 is now up and running. Please reach out to our flow cytometer specialist Dr. Beatriz Hernaez Estrada (bh663@drexel.edu) for training and consultation. Core-run sessions can be arranged at a case-by-case basis.

The flow cytometry can analyze up to 16 fluorescent parameters simultaneously. It is also equipped with small particle detector scatter that can detect small particles (as small as 90nm) and with the high throughput samples (HTS), which provides fully automated sample acquisition from 96-well U, V and flat-bottom plates and 384 well microtiter plates. The instrument is located in PISB room 410-f2.
Read More details on the instrument specifications and configuration
Questions? Email Dr. Beatriz Hernaez Estrada, Senior Research Scientist, School of Biomedical Engineering, at bh663@drexel.edu.
Research Compliance
ORI Regulatory Affairs and Research Compliance Training Update
The Office of Research and Innovation is pleased to announce that we are updating its educational resources and training programs to further support our commitment to research excellence and compliance. These updates are designed to ensure that all researchers, staff, and students have access to the most current information and best practices in research compliance and ethics.
REGISTRATION AVAILABLE (September 19, 2024)-Live RCR Course
Previously, face-to-face Responsible Conduct of Research (RCR) training, as required by NIH, was provided as Course RCRG 600, as assigned by Graduate Academic Advisors. Based on feedback from Faculty and Students and understanding the other commitments our students have during the year, we have chosen to move this to a single-day face-to-face course offered as part of Welcome Week, starting in the Fall of 2024. This course is being offered instead of the Winter RCR 600 course provided by the Graduate College. It is required for all individuals as assigned by their school, college or program, as well as all trainees, fellows, participants, and scholars receiving support through any NIH training, career development award (individual or institutional), research education grant, and dissertation research grant. Face-to-face RCR training is also strongly recommended for undergraduate and graduate level students, as well as faculty, who plan to conduct research, regardless of the funder, for individuals who have not otherwise completed live RCR training.
Training will be held on Thursday, September 19, 2024 from 8:30AM-4:30 PM. Individuals can register for RCR at the following link: Fall 2024 Responsible Conduct of Research (RCR) Training Registration. As physical space is limited, depending upon registration, we may ask individuals to switch to remote, if they are not a required attendee.
Please note that for programs requiring RCR 600, the Graduate College will contact the program managers directly to discuss how to accommodate this.
This change does not affect the Drexel University College of Medicine's RCR Course, School of Biomedical Engineering, Science and Health Systems RCR Course, or the RCR-CITI Training Requirements.
Development of Enterprise Learning: Research Compliance Course
ORI and HR have developed a learning module for all faculty and staff that provides essential training on Drexel University's Research Compliance and Regulatory Affairs programs and requirements.
This essential training is designed to ensure that everyone has a fundamental understanding of both Drexel University's commitments and our individual responsibilities, regardless of participation in research. This learning module will be deployed using the Career Pathway this Summer and will be in addition to the CITI Modules.
CITI Update-Drexel University & Salus University Merger
With Salus University's integration and the upcoming implementation of a new electronic system (COEUS Replacement Project), it's imperative that we align our operations and processes. As part of this effort, we are aligning the CITI (Collaborative Institutional Training Initiative) training requirement for all members of our research community.
CITI training provides essential education on responsible conduct of research, ethics, and regulatory compliance. It equips researchers with the knowledge and tools necessary to ensure that their work meets the highest ethical and professional standards. All individuals involved in research activities on behalf of Drexel and Salus University, including principal investigators, co-investigators, research assistants, graduate students, and any other personnel directly or indirectly engaged in research projects, must affiliate with Drexel University in the CITI system and complete any outstanding training modules by 11/01/2024.
Failure to complete CITI training by the 11/01/2024 deadline may result in an inability to start new research or cause existing research to expire, resulting in non-compliance.
To affiliate your existing CITI training and complete any outstanding modules, please follow the following instructions and training matrix:
For additional frequently asked questions, please review: Drexel University and Salus University CITI Affiliation Frequently Asked Questions [PDF].
Questions and Resources
We understand that your time is valuable, but the knowledge gained through these trainings for our Faculty, Staff and Students is crucial for maintaining the integrity and credibility of our research endeavors and institution. Your engagement and commitment to these requirements and the ethical conduct of research is appreciated.
Questions? Email Marisa Corbett, Executive Director of Research Quality Assurance, at marisa.jo.corbett@drexel.edu or Cassandra Myers, Associate Vice Provost of Research Compliance and Regulatory Affairs, at cassandra.j.myers@drexel.edu.
ORI Guidance And Procedure Update
As the Office of Research & Innovation (ORI) continues its efforts toward Drexel University's strategic plan and CLARITY project we are excited to introduce a new and updated SOPs and guidelines through ORI's established workgroup processes that will continue to provide best practices, enhance collaboration, and reinforce our commitment to Drexel University and our Research Community:
IACUC Procedure Updates
ACU-006 Assigning Pain and Distress Procedures [PDF]
- Purpose: Drexel University (DU) Institutional Animal Care and Use Committee (IACUC) has established this procedure to assist Principal Investigators in protocol preparation by providing guidelines for assigning research and teaching animals into appropriate pain and distress categories.
- Updates: This procedure has been revised with minor edits.
- A full list of revisions can be found in Section 7. of the procedure document.
- Update Effective: 4/24/2024
ACU-205 Sterilizing Surgical Materials Procedures [PDF]
- Purpose: To define the methods and documentation required for sterilizing surgical instruments and materials. Specific sterilization methods should be selected on the basis of the physical characteristics of the materials to be sterilized and sterilization indicators should be used to validate that materials have been properly sterilized.
- Updates: This procedure has been revised to include a title change for clarity. More information about chemical sterilization and hot bead sterilization usage. Methods NOT Acceptable for Sterilization have been added.
- A full list of revisions can be found in Section 4. of the procedure document.
- Update Effective: 4/24/2024
ACU-210 Genotyping Tissue Collection Procedures [PDF]
- Purpose: To provide guidance to the research community on the selection and execution of methods used to obtain mouse or rat tissue for genotyping purposes.
- Updates: This procedure has been revised and Distal Phalanx Biopsy Method (toe clipping) has been removed from the procedure.
- A full list of revisions can be found in Section 5. of the procedure document.
- Updates Effective: 4/24/2024
ACU-212 Non-Rodent Mammal Surgical and Post-Operative Care Procedures [PDF]
- Purpose: These procedures describe the proper procedures for surgery, including pre-operative and post-operative care of non-rodent mammals (such as rabbits, pigs) in accordance with the “Animal Welfare Act" and the Association for Assessment and Accreditation of Laboratory Animal Care, International's guidelines, as outlined in the "Guide for the Care and Use of Laboratory Animals."
- Updates: This procedure has been revised with minor edits which include the following: Change from should to must in “All surgical records must include the following information.” An Anesthesia and Surgical Log has been added to end of the procedure document. Clarification that the animal must be continuously monitored with written observations made at least every 15 minutes until it regains sternal recumbency.
- A full list of revisions can be found in Section 6. of the procedure document.
- Updates Effective: 5/22/2024
ACU-214 Expired Drugs and Medical Materials Procedures [PDF]
- Purpose: The purpose of these procedures is to provide guidance to principal investigators and their staff regarding the expiration and disposal of expired drugs and medical materials. These procedures have been developed to ensure drugs and materials used in laboratory animal research are safe and effective and to ensure compliance with the Guide for the Care and Use of Animals, 8th Edition, and the NIH Guidelines and the PHS Policy on the Humane Care and Use of Laboratory Animals.
- Updates: This procedure has been revised to include clarifications for how to interpret manufacturer expiration dates, clarifications to ensure the day is included in the preparation and expiration date of dilutions and mixtures, and clarifications that vials of sterile saline and sterile water follow the same expiration guidance as bags of fluids.
- A full list of revisions can be found in Section 11. of the procedure document.
- Updates Effective: 5/22/2024
ACU-217 Sanitizing Equipment for Use with Research Animals Procedures [PDF]
- Purpose: To define the methods and documents required for sanitization of equipment, instruments, and the experimental environment for use in animal research by the proper use of cleaning and disinfectants. The goal of these procedures is to ensure clean equipment, instruments, and experimental
environments for research animals and to prevent the spread of infectious agents within the animal colony. - Updates: This procedure has been revised to include minor edits.
- A full list of revisions can be found in Section 7. of the procedure document.
- Updates Effective: 5/22/2024
- IMPORTANT: The efficacy of the sanitation process should be verified periodically by microbiological monitoring, or other appropriate methods and the data reviewed to ensure the effectiveness of these methods. ULAR can monitor the effectiveness of the procedure by swabbing representative equipment after the sanitization process. Monitoring the effectiveness of sanitization should be done annually to comply with the Guide for the Care and Use of Laboratory Animals. As we prepare for the AAALAC site visit this fall, please contact ULAR to conduct monitoring of the sanitation process of your equipment.
ACU-218 Tumor-Bearing Animal Procedures [PDF]
- Purpose: Drexel University Animal Care and Use Committee has established these procedures to identify and resolve animal welfare issues related to experimentally-induced tumors in animals.
- Updates: This procedure has been revised to include the addition of growth charts for two commonly used species and resource link to Charles River Laboratories. The addition of “The use of imaging (IVIS or ultrasound) can be used to determine the overall tumor burden.” has also been added.
- A full list of revisions can be found in Section 5. of the procedure document.
- Updates Effective: 5/22/2024
ACU-206 Animal Blood Collection Sites [PDF]
- Purpose: The Drexel University Animal Care and Use Committee has established these procedures to specify the common blood collection routes consistent with The Guide for the Care and Use of Laboratory Animals and AVMA Guidelines on Euthanasia of animals. These procedures will ensure that distress to the animal is minimized during blood collection.
- Updates: This procedure has been revised with minor edits which include the removal of tail clip collection route as a common method of blood collection for rats.
- A full list of revisions can be found in Section 5 of the procedure document.
- Updates Effective: 6/26/2024
ACU-207 Animal Blood Volume Sampling Procedures [PDF]
- Purpose: The Drexel University Animal Care and Use Committee has established these procedures to specify blood sampling volumes which can be obtained without having an adverse effect on research animals.
- Updates: This procedure has been revised with minor edits including the change in timeframe from a 28-day period to a 14-day (two week) period where a single blood sample must not exceed 10% of blood volume.
- A full list of revisions can be found in Section 4 of the procedure document.
- Updates Effective: 6/26/2024
ACU-209 Animal Euthanasia Procedures [PDF]
- Purpose: The Drexel University Institutional Animal Care and Use Committee (IACUC) has established this procedure to assure compliant and humane methods of euthanasia consistent with The Guide for the Care and Use of Laboratory Animals and AVMA Guidelines on Euthanasia [PDF].
- Updates: This procedure has been revised to include multiple edits. Notable edits include:
- Removal of “Anesthetic Overdose, Desiccator Jar/Open Drop” as a recommended euthanasia method. The use of this euthanasia method must be justified and approved by the IACUC before use.
- Addition of “MS222 solutions for tank immersion and injections are acidic and irritating and must be buffered with sodium bicarbonate to a physiologically appropriate pH before use.” “MS-222 stock solutions should be utilized the same day as preparation per vendor recommendation.” “Special handling and preparation of MS222 required because it is a chemical hazard and requires biosafety approval prior to use.”
- Addition of the compassion fatigue resource: AALAS COST OF CARING: RECOGNIZING HUMAN EMOTIONS IN THE CARE OF LABORATORY ANIMALS
- A full list of revisions can be found in Section 7 of the procedure document.
- Updates Effective: 6/26/2024
ACU-215 Physical Restraint Procedures [PDF]
- Purpose: To assure appropriate IACUC oversight of physical restraint of animals and to ensure humane care and use of animals when such restraint is employed.
- Updates: This procedure has been revised with minor edits.
- A full list of revisions can be found in Section 6 of the procedure document.
- Updates Effective: 6/26/2024
We want to thank the IACUC members who participated in the review process, and their dedication and commitment were instrumental in the success we achieved. ORI is truly thankful to have had the opportunity to work alongside you.
These documents are available on the Animal Care and Use Guidelines and Procedures page. Stay up to date on all animal care and use procedure updates on the Post-Approval Monitoring "The Latest" page.
Questions? Email Liz Hann, Director of Animal Welfare, at iacuc@drexel.edu.
Institutional Biosafety Committee Updates
The Institutional Biosafety Committee (IBC) has revised all biosafety protocol application forms to provide greater clarity, create consistency, and incorporate recent updates from the NIH Guidelines (HTML and PDF).
Notable changes include:
- Required reporting on the use of gene drive modified organisms (GDMOs). This is a new requirement under the NIH Guidelines. If your work involves GDMOs, contact the IBC (biosafety@drexel.edu) for guidance on performing a comprehensive risk assessment and conducting your work using appropriate biological containment practices.
- Addition of a form to document a PI change. In circumstances where a different PI is assuming responsibility for investigations previously approved by the IBC, the change must be documented using Form G: Change of PI Amendment. An amendment involving a PI change will be reviewed by the full committee at its next meeting.
The revised forms are available on the University Biosafety website. The use of the new application forms will be effective for our July meeting cycle. The use of the forms is required moving forward. Any submissions using forms from 2017 will need to be resubmitted using the new forms.
We want to thank the IBC members who participated in the protocol application form review process, and their dedication and commitment were instrumental in the success we achieved. ORI is truly thankful to have had the opportunity to work alongside you.
Questions? Email Liz Hann, Director of Animal Welfare, at biosafety@drexel.edu.
White House Releases Final Guidelines for Implementing NSPM-33
In response to growing security threats, On July 9, 2024, the White House's Office of Science and Technology Policy (OSTP) has finalized its guidance for implementing National Security Presidential Memorandum-33 (NSPM-33), including those in higher education.
The OSTP guidance and NSPM-33 require institutions to implement comprehensive security measures within 18 months, including:
- Implement a cybersecurity program consistent with cybersecurity requirements for research institutions described in the CHIPS and Science Act, within one year after NIST of the Department of Commerce publishes that resource.
- Established requirements for foreign travel security, including training and a travel reporting program
- Export Control and Research Security Training, including certification that the institution requires covered individuals to complete training on compliance requirements
Drexel University's ORI, including the Export Control team, as well as Drexel Global, IT, OGC, HR, and other key stakeholders have already initiated the implementation of this work including ongoing activities:
- Integrated and revised the “International Travel Registration” process to include export control and research compliance review where applicable.
- Faculty traveling internationally, utilizing a p-card or being reimbursed from Drexel (departmental or research funds)
- Students conducting international co-ops, conducting research internationally, studying abroad
- c. Staff traveling internationally conducting Drexel business
- Implemented CITI Export Compliance training for specified users, and inclusion of export, travel, and research security in the upcoming Research Compliance Course being released through Enterprise Learning.
- Where applicable in research Export Control has added “Undue Foreign Influence” CITI training
- Inclusion of Foreign Talent Disclosure in Research Conflict of Interest Disclosure and International visitor/sponsorship forms.
- Establishment of a Research Security Tier and Framework Workgroup to establish a comprehensive and effective research security tier structure to enhance the protection of research data and ensure compliance with regulations and requirements.
Read More about updated guidance on Fedscoop
Questions? Email Lacee Harris, PhD, Executive Director of Research Compliance, at lacee.harris@drexel.edu, Elan Mitchell-Gee, PhD, Director, Export Control and Research Security, at elan.virginia.mitchell-gee@drexel.edu, or Cassandra Myers, Associate Vice Provost of Research Compliance and Regulatory Affairs, at cassandra.j.myers@drexel.edu.
Research Development
Introducing the Research Development team
The Research Development Team, under the auspices of the Office of Research and Innovation, is poised to debut its inaugural services to the research community at Drexel. Led by Gwynne Grasberger, Associate Vice Provost, Research Development, the muti-disciplinary team of seasoned professionals has incorporated business development concepts and practices into their unique approach and has capitalized on the expertise of acknowledged experts in the burgeoning field of Research Development.
After developing and copywriting their Research Development Lifecycle, the stand-up team has spent the last year listening and responding to researchers, department heads, and ADRs to fine tune and customize their inaugural services, with the goal of building capacity and competency in the pursuit of federal funding for research endeavors.
Research Development has piloted their methodology with four AEO teams through co-created project plans. Quarterly check-ins with the teams have built momentum in networking, connecting, and sharing science among research collaborators. Lessons learned in this process will help form the eventual full complement of research development services and resources when the team becomes fully operational.
More information on the team and its offerings
Questions? Email researchdevelopment@drexel.edu.
Sponsored Programs
Ongoing sponsor updates can be found on our sponsor page, in addition to the newsletter.
NSF Updates
NSF recently revised its Proposal & Award Policies & Procedures Guide (PAPPG) (NSF 24-1) for proposals submitted or due on or after May 20, 2024.
Key Changes Include:
- Biographical Sketch and Current and Pending (Other) Support
- The revised format (Common Forms) are in SciENcv and must be used for proposals submitted or due on or after May 20, 2024. Both documents are required for all senior/key personnel.
- Research.gov and Grants.gov will generate a compliance error message if a proposer or awardee attempts to upload a prior version of either the biographical sketch or the current and pending (other) support format or a PDF not generated in SciENcv.
- The Research.gov Project Reporting System will also enforce use of the revised current and pending (other) support format when uploaded in annual and final annual project reports.
- Research.gov and Grants.gov will no longer enforce a page limitation for the biographical sketch per PAPPG Chapter II.D.2.h.(i).
- Synergistic Activities
- The biographical sketch will no longer contain a synergistic activities section. A one-page synergistic activities document will be created for each individual designated as senior/key personnel as part of the required senior/key personnel documents in Research.gov and Grants.gov. See PAPPG Chapter II.D.2.h(iv)< for additional information.
- Malign Foreign Talent Recruitment Program Certifications
- All senior/key personnel must certify that they are not a party to a Malign Foreign Talent Recruitment Program per PAPPG Chapter II.D.1.e(ii). This new certification is included on both the biographical sketch and current and pending (other) support forms in SciENcv.
- An Authorized Organizational Representative (AOR) must certify that all senior/key personnel have been made aware of and complied with the requirement that they are not a party to a Malign Foreign Talent Recruitment Program at the proposal submission per PAPPG Chapter II.D.1.d(ix).
- Mentoring Plan
- The Mentoring Plan requirement has been expanded to include graduate students. Research.gov and Grants.gov will enforce that a Mentoring Plan not to exceed one page is uploaded for proposals when there is funding for graduate students and/or postdoctoral scholars. Refer to PAPPG Chapter II.D.2.i(i).
- Additional Updates
- Three new products have been enabled in annual and final annual project reports in Research.gov: Interventions (e.g. clinical or educational), New Business Creations, and Training and Professional Development Materials or Courses.
- A new Individual Development Plan certification has been added as part of the annual reporting process to certify that each graduate student or postdoctoral scholar has an Individual Development Plan. This certification is completed by the Principal Investigator (PI) or co-PI in Research.gov.
NSF Resources:
- Summary of Changes to the PAPPG (NSF 24-1)
- NSF Proposal & Award Policy Updates (NSF 24-1) webinar
- NSF Biographical Sketch and Current and Pending (Other) Support websites
- NSTC Pre-award and Post-award Disclosures Relating to the Biographical Sketch and Current and Pending (Other) Support [PDF]
- Implementing the Common Forms for the Biographical Sketch and Current and Pending (Other) Support (webinar)
Effective July 1, 2024, NSF added six new product types in the NSF Public Access Repository (NSF-PAR): audiovisual, data paper, educational aid and curriculum, posted content, software, and sound. PIs and co-PIs can manually add these product types to project reports in Research.gov. For more information, please review the Research.gov About Public Access page for FAQs and updated how-to guides that include the six new product types. For the latest information on open science, please visit the NSF Public Access Initiative website.
Questions? Email Sarah Saxton, Assistant Vice Provost, Sponsored Programs, at sarah.m.saxton@drexel.edu.
NIH Updates
NIH is increasing the childcare support to $3,000 for applicable NRSA individual fellowships and institutional training awards effective with FY2024 awards. Please refer to NOT-OD-24-116 for more information.
NIH has published a revised NIH Grants Policy Statement for FY2024. The revised policy applies to all NIH grants and cooperative agreements with budget periods beginning on or after October 1, 2023. All changes in regard to the updated Uniform Guidance (2 CFR Part 200) that will be implemented on October 1, 2024 will be included in the FY2025 release of the NIH Grant Policy Statement. For more information, please refer to NOT-OD-24-115.
Please remember that effective May 25, 2024, markups in Resubmission applications are no longer allowed. Changes made to the Resubmission application would only be outlined in the Introduction attachment. Please refer to NOT-OD-24-061 for further information.
For RPPRs submitted on or after October 1, 2024, NIH will have additional questions to align with the NIH Final Policy on Data Management and Sharing, including updates on the status of data sharing, repositories and unique identifiers for data that have been shared. Please refer to NOT-OD-24-123 for additional information. A revised RPPR Instruction Guide will be posted to the Research Performance Progress Report (RPPR) NIH webpage once approved.
NIH will have many changes to grant applications submitted for due dates on or after January 25, 2025 (NOT-OD-24-084).
Key Changes Include:
- Simplified Review Framework for Most Research Project Application Grants
- The National Institutes of Health (NIH) is simplifying the framework for the peer review of most Research Project Grant (RPG) applications, effective for due dates on or after January 25, 2025. These changes are designed to address the complexity of the peer review process and mitigate potential bias.
- Revisions to the NIH Fellowship Application and Review Process
- Fellowship applications submitted on or after January 25, 2025 will follow a revised application and review criteria. The goal of the changes is to improve the chances that the most promising fellowship candidates will be consistently identified by scientific review panels.
- Several changes to the application include:
- Grades will no longer be required or allowed on the Candidate biosketch.
- ii. Candidates will be required to submit four personal statements: 1) professional and fellowship goals, 2) fellowship qualifications, 3) self-assessment, and 4) scientific perspective
- Revisions to the research training plan sections: heading revisions and moving the Selection of Sponsor and Institution into another area of the application
- Sponsors and Co-sponsors will be required to submit five statements: 1) mentoring approach and candidate mentoring plan, 2) prior commitment to training and mentoring, 3) commitment to the candidate's research training plan, 4) research training environment, and 5) candidate's potential. If a candidate is proposing to gain experience in a clinical trial, a sixth statement on clinical training will be required.
- Updated Reference Letter Instructions for Referees
- NIH is updating the instructions for reference letters to provide more structure so letters will better assist reviewers in understanding the candidate's strengths, weaknesses, and potential to pursue a productive career in biomedical science. The submission process will remain unchanged.
- Updates to NRSA Training Grant Applications
- Three key changes to the application
- The Recruitment Plan to Enhance Diversity will be its own attachment in the PHS 398 Research Training Program Plan Form.
- Mentor training expectations will be more clearly defined int the parent T32 Notice of Funding Opportunity (NOFO).
- Institutional Training data tables will be updated to reduce burden and promote consistent information collection across training programs.
- Tables 1 & 2: Applicants will be expected to provide data only for the training stage(s) reflected in the proposed program.
- Table 5 (Publications of Those in Training): Table will be reorganized so that the first column is the trainee, not faculty member, and applicants will be allowed to include interim research products to which the trainee contributed.
- Table 6 (Applicants, Entrants, & their Characteristics for the Past 5 Years): No longer will ask for trainee characteristics related to prior academic and research experience.
- Table 8 (Program Outcomes: Predoctoral and Postdoctoral): Part II "Those Clearly Associated with the Training Grant” will not be included.
- NIH will now include “Training in the Responsible Conduct of Research” and “Recruitment Plan to Enhance Diversity” as items that contribute to the overall impact score. These items will move from “Additional Review Considerations” and will be included as “Additional Review Criteria.” As such reviewers will evaluate the “Training in the Responsible Conduct of Research” and the “Recruitment Plan to Enhance Diversity” while determining scientific and technical merit, and in providing an overall impact score.
- Three key changes to the application
- Updated Application Forms (FORMS-I)
- all applications submitted for due dates on or after January 20, 2025 will need to be submitted using the new application forms.
- Most changes will be seen in the PHS 398 Research Training Program Plan, PHS Fellowship Supplemental Form, and Training Data Tables due to the revisions of the NRSA Training Grant and NIH Fellowship Application changes.
- Common Forms for Biographical Sketch and Current and Pending (Other) Support
- NIH is adopting the Biographical Sketch Common Form and the Current and Pending (Other) Support Common Form in 2025. The Common Forms represent a collaborative effort between Federal research agencies to ensure standard disclosure requirements as outlined in the National Security Presidential Memorandum - 33.
NIH Resources:
- Simplified Review of Research Project Grant Applications Webpage
- Recorded Webinar: NIH's Simplified Peer Review Framework for NIH Research Project Grant (RPG) Applications and Impact to New and Existing Funding Opportunities
- NOT-OD-24-085: Simplified Review Framework for NIH Research Project Grant Applications - Update and Implementation Plans
- NOT-OD-24-010: Simplified Review Framework for NIH Research Project Grant Applications
- NOT-OD-23-034: Request for Information (RFI) on Proposed Simplified Review Framework for NIH Research Project Grant Applications
- Revisions to the NIH Fellowship Application and Review Process Webpage
- September 19, 2024 Webinar Registration: Revisions to the Fellowship Application and Review Process
- NOT-OD-24-107: Implementation of Revisions to the NIH and AHRQ Fellowship Application and Review Process
- NOT-OD-23-110: Request for Information (RFI) on Recommendations for Improving NRSA Fellowship Review
- Reference Letters Webpage (new instructions will be posted fall 2024)
- Recorded Webinar: Updates to NIH Training Grant Applications
- Updates to NIH Institutional Training Grant Applications Webpage
- NOT-OD-24-129: Updates to NIH Institutional Training Grant Application for Due Dates on or after January 25, 2025
- High Level Summary of FORMS-I Changes Webpage
- NOT-OD-24-086: New NIH “FORMS-I” Grant Application Forms and Instructions Coming for Due Dates on or after January 25, 2025
- Changes Coming to NIH Applications and Peer Review in 2025 Webpage
- Video Overview of Grant Application and Review Changes for Due Dates on or after January 25, 2025
- Biosketch Format Pages, Instructions and Samples Webpage (to be updated)
- Other Support Webpage (to be updated)
Questions? Email Sarah Saxton, Assistant Vice Provost, Sponsored Programs, at sarah.m.saxton@drexel.edu.
NASA Updates
NASA plans to implement the NSTC common forms for the Biographical Sketch and Current and Pending (Other) Support starting on October 1, 2024, for NASA grants and cooperative agreements. NASA states in the notice they plan to implement the common forms “with minor deviations” and it is unclear from the notice what those will be. NASA will have a table entitled “NASA Pre-award and Post-award Disclosure Requirements,” which will provide reference information regarding pre-award and post-award disclosures. NASA is proposing a unique definition for Senior/key persons as all Principal Investigators (PIs), all co-Principal Investigators (CoPIs), and co-Investigators (Co-Is) proposing to spend 10 percent or more of their time in any given year on a NASA-funded grant or cooperative agreement. To comply with CHIPS and Science Act of 2022, which requires certifications that senior/key personnel are not a party to a malign foreign talent recruitment program, NASA will collect certification but will require award recipients to maintain original forms with digital signatures and make them accessible to NASA in accordance as digital signatures are not retained in their system (NSPIRE).
Questions? Email Sarah Saxton, Assistant Vice Provost, Sponsored Programs, at sarah.m.saxton@drexel.edu.
OMB Guidance for Federal Financial Assistance Update
The Office of Management and Budget (OMB) is revising its guidance (including Part 200: Uniform Guidance) for the purpose of: (1) incorporating statutory requirements and administration priorities; (2) reducing agency and recipient burden; (3) clarifying sections that recipients or agencies have interpreted in different ways; and (4) rewriting applicable sections in plain language, improving flow, and addressing inconsistent use of terms within the guidance. OMB's revisions are intended to improve Federal financial assistance management, transparency, and oversight through more accessible and easily understandable guidance. The effective date for the final guidance is October 1, 2024. For more information, please review the Federal Register.
Key changes include:
- Equipment-Related Thresholds [200.313]: Increases the acquisition value threshold relevant to the equipment definition from $5,000 to $10,000.
- Change is either made in a rate proposal and effective with the new rates negotiated OR changed with an impact statement submitted with a rate extension request.
- De minimis Indirect Cost Rates [200.414]: increased from 10% to 15% over the modified total direct costs (MTDC) In addition, pass-through entities must accept all federally negotiated indirect cost rates for subrecipients.
- Modified Total Direct Costs [200.1]: Increased definition threshold for up to $50,000 (previously $25,000) of each subaward (regardless of the period of performance of the subaward under the award).
- Change is either made in a rate proposal and effective with the new rates negotiated OR changed with an impact statement submitted with a rate extension request.
- Fixed Amount Awards and Subawards [200.333]: At the conclusion of the award, it is required to identify activities that were not completed. Fixed amount subawards increased from $250,000 to $500,000 without prior approval from federal agency.
Questions? Email Sarah Saxton, Assistant Vice Provost, Sponsored Programs, at sarah.m.saxton@drexel.edu.
NSF Spring 2024 Grants Conference
Did you miss the Spring 2024 NSF Grants Conference held in June? Fortuantely, NSF's Policy Office Outreach has uploaded all recorded conference sessions to their website for the research community to view.
Watch the recorded NSF Grants Conference Sessions
Questions? Email Sarah Saxton, Assistant Vice Provost, Sponsored Programs, at sarah.m.saxton@drexel.edu.
Updated FAQs for Linked Fund Numbers on Collaborative Research Awards
Since the implementation of the Linked Fund Number template and process, additional questions have come up from the research community. In order to provide more clarity, the FAQs have been updated on the ORI website.
Questions? Email Sarah Saxton, Assistant Vice Provost, Sponsored Programs, at sarah.m.saxton@drexel.edu.
Updated Facilities and Administrative (F&A) Rates for Sponsored Programs
Drexel University has finalized its Facilities and Administrative (F&A) rate negotiation with the Department of Health and Human Services' Cost Allocation Services. The new rate agreement is for FY2024-2027. One significant change is the Department of Defense (DoD) increased the administrative component of the indirect cost rate to 28% on DoD contracts and subcontracts. A summary of the F&A rates along with a copy of the Negotiated Indirect Cost Rate Agreement (NICRA) can be found on Drexel's Office of the Comptroller's website.
Questions? Email Sarah Saxton, Assistant Vice Provost, Sponsored Programs, at sarah.m.saxton@drexel.edu.
Fiscal Year 2025 Approved Fringe Rates for Sponsored Projects
Drexel University has finalized the negotiations of the fiscal year 2025 fringe benefit rates for sponsored projects with the U.S. Department of Health and Human Services (DHHS). The finalized rates remain the same as the proposed rates. COEUS and Banner have been updated to reflect the new proposed rates effective July 1, 2024. FY2025 Fringe Benefit Rates and the FY2025 Fringe Benefit Rate Breakdown can be found at the Research Accounting Services section of the Office of the Comptroller's website.
Questions? Email Sarah Saxton, Assistant Vice Provost, Sponsored Programs, at sarah.m.saxton@drexel.edu.
Subawards and Contracts Update
The Subawards Team has fully transitioned to three full-time consultants who working on executing all outgoing subawards in addition to reviewing current processes and procedures and developing guidelines. All new subaward negotiations are being entered into Coeus and can be reviewed in the Negotiations tab to provide transparency of the subaward status. If you have a question about the current status of an outgoing subaward, please reach out to subawards@drexel.edu. To request a new subaward or modification, please reach out to your Grants Administrator to start the process.
Lois Anderson, Contracts Administrator, left Drexel University on June 28, 2024 to pursue another opportunity. OSP thanks her for her dedication to the Contracts Team. OSP is currently starting the hiring process for a new Contracts Administrator. During this transition process, please note that there may be some delays in executing contracts and agreements. If you have any questions concerning a particular matter, please reach out to Alice Reuther, Director, Contracts and Subawards at alice.susana.reuther@drexel.edu.
Questions? Email Sarah Saxton, Assistant Vice Provost, Sponsored Programs, at sarah.m.saxton@drexel.edu.
Did You Know?
Did You Know About the ORI's Research Business Operations & Technology (RBOT) Team?
The Research Business Operations & Technology (RBOT) team was formed in 2023 by merging the ORI Finance & Administration (F&A) team with the ORI Research Systems & Analytics (RS&A) to create a single administrative entity with increased alignment and efficiency. Our mission is to provide the foundation for operational and cultural excellence within the ORI.
The RBOT team focuses on operational support for ORI's functional teams, as well as University research reporting, COEUS and DragonSPOT support and training, and system implementation/management (COEUS Replacement Project). Please see updates on some of our recent work below:
COEUS Replacement Project (CRP)
We've made great progress preparing for the kickoff of our Novelution implementation. Contracting is moving along, and we are engaged with the vendor on project planning and other preparations. We're currently projecting a mid-September start date and look forward to the vast improvements the new system will bring to all members of the Drexel research community! Please feel free to reach out to Doug Stay at douglas.r.stay@drexel.edu with any questions.
ORI Clinical Research Services
ORI's RBOT team offers payment options for Drexel clinical and non-clinical research studies through two different payment platforms, Clin Card and Concourse. Clin Card is used for clinical research studies in conjunction with the University's CTMS (Clinical Trial Management System), Clinical Conductor. Concourse is managed through a partnership with JP Morgan Chase and can be used for both clinical and non-clinical research studies.
Drexel has leveraged the Clin Card payment platform for over 8 years by providing a simple, non-cash option for participant payments. Participants receive a debit card loaded with their incentive payments for each visit they complete in a study. Once activated and loaded, the participant can use it like a debit card.
The University has used the cashless payment platform Concourse (formerly known as Quick Pay) since 2020. Concourse has grown tremendously throughout Drexel since its implementation. There are currently 108 studies utilizing the system to manage incentive payments and Community Advisory Board Members (CAB) stipends. After initial setup of a secure account with JP Morgan Chase, study participants and CAB members receive ACH deposits into the bank account of their choice.
To learn more about these cashless payment options for your clinical and non-clinical research studies please contact Jeannine Reed-Heil in RBOT at jeannine.reed-heil@drexel.edu.
Questions? Email Doug Stay, Assistant Vice Provost, Research Business Operations & Technology, at douglas.r.stay@drexel.edu.
