BD FACSymphony A1 – Flow Cytometer

Flow cytometry is widely used in areas of research that require analysis or isolation of cells from suspension. This technology makes use of fluorescent probes targeted to specific cell-associated molecules to characterize the diversity and function of complex cell populations.
CIC’s flow cytometer BD FACSymphony A1 is located in PISB room 410-f2. We provide flow cytometry services for analysis of cells, as well as training and consultation for conducting and planning experiments. Please contact Dr. Beatriz Hernaez Estrada for training or more information.
The BD FACSymphony A1 flow cytometer is an air-cooled multi-laser benchtop flow cytometer with the ability to acquire 16 fluorescent parameters. BD FACSymphony A1 cell analyzer is also equipped with small particle detector scatter that can detect small particles such as extracellular vesicles as small as 90 nm and with the high throughput sampler (HTS), which provides fully automated sample acquisition from 96-well U,V, and flat-bottom plates as well as 384-well microtiter plates.
Technical Specifications
Laser lines
- 405 nm
- 488 nm
- 561 nm
- 637 nm
| Detector | LP mirror | BP Filter |
Fluorochromes |
|---|---|---|---|
| A | 750 | 780/60 |
BV786 |
| B | 690 | 710/50 |
BV711 |
| C | 655 | 670/30 |
BV650 |
| D | 595 | 610/20 |
BV605 |
| E | 505 | 525/50 |
BV480 |
| F | 410 | 450/50 |
BV421 V540 |
| F | 410 | Optional: 431/28 | - |

| Detector | LP mirror | BP filter | Fluorochromes |
|---|---|---|---|
| B | 690 | 710/50 | BB700 PerCP-Cy5.5, 7-AAD |
| C | 505 | 530/30 | BB515, AF488, FITC |
| E | - | 488/10 | SSC |
| F | - | - | SP SSC (*Small particle detector) |

| Detector | LP mirror | BP filter | Fluorochromes |
|---|---|---|---|
| A | 750 | 780/60 | PE-Cy7 |
| B | 690 | 710/50 | PE-Cy5.5 |
| C | 655 | 670/30 | PE-Cy5 |
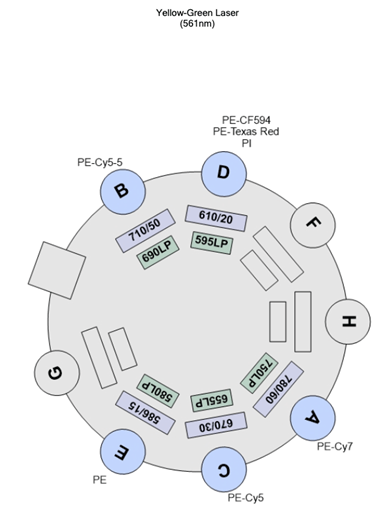
| D | 595 | 610/20 | PE-CF594 PE-Texas Red PI |
| E | 505 | 586/15 | PE |
| Detector | LP mirror | BP filter | Fluorochromes |
|---|---|---|---|
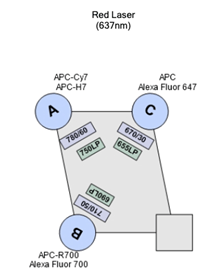
| A | 750 | 780/60 | AP-Cy7 APC-H7 |
| B | 690 | 710/50 | APC-R7 Alexa Fluor 700 |
| C | 655 | 670/30 | APC AF647 |
BD High Throughput Sampler (HTS)
Provides rapid, fully automated sample acquisition from 96-well U, V and flat-bottom plates as well as 384-well microtiter plates. BD FACSDiva software allows you to create customized delivery protocols with user defined mixing, wash, and analysis parameters. In high-throughput mode, the HTS can process a 96-well plate in fewer than 15 minutes. Standard mode can be selected for acquisition of larger sample volumes.
Note: Do not use the HTS if you need to analyze every cell in your sample because the HTS system has a dead volume and uses part of the sample to prime. Injected sample volume is discarded once the stopping criteria is reached. There is no way to get the sample back once the sample is aspired.
Using the HTS option requires a separate training please contact Dr. Beatriz Hernaez Estrada for more information.
Small particle detection
Our flow cytometer enables the detection of small particles as small as 90 nm polystyrene beads. This includes extracellular vesicles, viral particle, exosomes
Using the small particle detection option requires a separate training please contact Dr. Beatriz Hernaez Estrada for more information.
Software
The analysis workstation will be available for researchers who want to use FlowJo for data analysis. Training will be provided to the investigators based on their needs.
Flow cytometer availability
- The flow cytometer is accessible to trained users 24/7
- Note that trained users are welcome to sign up for 4 hour time blocks between the hours of 9 am and 6 pm daily or for more than 4 hours during weekends and off hours.
- Assisted use and trainings take place Monday through Friday during regular working hours by appointment.
- Staff is available on a limited basis during off-hours to help troubleshoot instrument issues.

Introduction to flow cytometry
Flow cytometry is a biophysical technology used for cell counting and biomarker detection within a heterogeneous population. It simultaneously measures and analyses multiple physical and chemical characteristics of particles, usually cells, as they flow in a fluid stream through a beam of light. Any suspended particle or cell from 0.2–150 micrometers in size is suitable for analysis, and up to thousands of particles per second can be analyzed. Cells from tissues must be disaggregated before analysis.
There are two main physical parameters that are used in flow cytometry: light scattering and fluorescence.

Flow Cytometer light scattering
1) Light scattering
Light scattering occurs when a particle deflects incident laser light. Forward-scattered light (FSC) is a measurement of the light diffracted by the particle and is proportional to its size. Side-scattered light (SSC) is a measurement of refracted and reflected light from the particle. SSC is proportional to cell granularity and internal complexity.
Correlation between FCS and SCC can be used to differentiate between different cell types in a heterogeneous cell population.
For instance, the figure on the right shows how light scattering can be used to differentiate between leucocyte subpopulations in the blood. As opposed to neutrophils, lymphocytes are small leucocytes with low internal complexity.
2) Fluorescence
Fluorochromes, such as fluorescently-labelled antibodies, can be used to detect target proteins. Cell types or subpopulations within a cell type can therefore be differentiated by labelling multiple cell markers.
For instance, the cell surface proteins known as clusters of differentiation (CD) are widely used in immunology to differentiate between different cell subpopulations. The example provided below shows how lymphocytes, differentiated from other blood cells by light scattering, can be further differentiated into subpopulations by fluorescence based on the surface expression of CDs (CD3, CD16, and CD19).


