Translational Research
Multiple research opportunities are available for surgical residents interested in any of the described research areas.
Portable Breast Cancer Screener
The Department of Surgery is collaborating with the Departments of Material Science and Engineering, Pathology and Laboratory Medicine, and the School of Biomedical Engineering in the development of a low-cost portable breast cancer screening device. Breast cancers are stiffer than normal breast tissues and the differences in stiffness between cancer and normal tissues can be exploited for early detection. We have developed a piezoelectric-based tissue stiffness measurement/detecting system which we have named the Piezoelectric Finger (PEF). The PEF can measure both the elastic modulus (E) (the stiffness of tissue under compression) and shear modulus (G) (the stiffness of tissue under shear) with great precision. Using our prototype device and ex vivo breast samples we showed that the contrast between the E or G of abnormal breast tissues such as carcinoma in situ (CIS) and invasive carcinoma (IC) versus that of normal breast tissues consisting of glands and fat was 3-5 fold for women of all age groups. This technology is targeted for developing countries which do not have the resources to implement programs similar to the mammography based screening program available in the United States.
Participating faculty:
Additional information is available at sensor.materials.drexel.edu/
Back to Top
Quantum Dots for Tumor Margin Detection
Breast cancer is increasingly being diagnosed at an early stage allowing treatment with breast conserving surgery. After excision of the tumor it is important to determine that the tumor margin is clear of any cancer cells. If tests determine that the margin is not clear, the patient must return for re-excision. Currently there is no reliable method to assess margins intra-operatively.
The development of a reliable intra-operative test that allows the surgeon to test for residual cancer cells rapidly after the initial excision can allow the re-excision to occur immediately during the initial surgery reducing overall treatment costs and pain and inconvenience to the patient. Quantum Dots (QDs) are nanocrystals that fluoresce over a long period of time without photobleaching. QDs are brighter and therefore more sensitive than traditional fluorescent molecules, permitting imaging of far fewer cells. Dr. Wei-Heng Shih and Dr. Wan Shih have developed an approach for making highly luminescent aqueous QDs using an environmentally friendly aqueous process from which QDs can be directly conjugated for bioimaging. Through conjugation with appropriate antibodies these QDs can be used to image cancerous cells in excised tissues to allow timely examination of margin clearance. The intensity of the photoluminescence (PL) will indicate if cancer cells are on the excised tissue surface.
Participating faculty:
Additional information is available at sensor.materials.drexel.edu/
Back to Top
Wound monitoring, therapy, and regenerative medicine
Dr. Michael Weingarten (View Profile) is involved with several research projects related to wound care and novel uses of technology to augment wound healing.
"Wound-Healing Device Trial"
Pulse (Summer 2017)
Back to Top
Near Infrared Wound Monitor
Wound assessment is highly subjective and can be misleading. The predominant metric to assess wound healing is a decrease in wound size. However, it has be shown in the scientific literature that shrinkage in wound size only has a 58% correlation to actual healing. Optical properties of wound tissue and surrounding environment reflect metabolic activity and correlate with wound healing. Using a Near Infrared Spectroscopy (NIRS) developed by the Drexel University School of Biomedical Engineering, we have discovered that the time course of the absolute amount of oxygenated hemoglobin in tissue is a strong indicator of wound healing progress. Based on measurements of the oxygenated and deoxygenated hemoglobin using the NIRS device, a wound healing index is computed which provides the physician with the status of healing in the wound. This device is currently undergoing human subject testing and technical refinement.
Participating faculty:
Back to Top
Bioactive Alimentary Protein-based Scaffolds (APS) for Wound Healing and Regenerative Medicine
There is an unmet clinical need for simple, first-response bioactive wound dressings that would help reduce the morbidity and mortality from severe injury to the skin. Our long-term goal is to develop and commercialize a novel bioactive engineered acellular wound dressing, "Alimentary Protein Based Scaffolds" (APS), which will have multiple applications for wound healing treatments and regenerative medicine. Our scaffold is produced by electrospinning of a soybean protein base. Our initial experiments involve demonstrating that the APS will accelerate wound healing in an animal model. We envisage that APS, packaged as an acellular scaffold, will be sterile, portable, and readily available, with an extended shelf life.
Participating faculty:
Back to Top
Acousto-optic Theragnostic Approach for Chronic Wound Management
The long-term objective of our proposed program is to develop a non-invasive chronic wound treatment that combines optic and acoustic modalities in a synergistic way. This theragnostic, i.e., thera(peutic) and (dia)gnostic technology, merging a non-invasive ultrasound therapy with Near Infrared (NIR) diagnostic monitoring, will allow a wound care provider to prescribe low frequency ultrasound therapy through a "Band-Aid®"-like wearable patch, assess the status of wound healing with digital imaging and NIR, and adjust the ultrasound parameters as necessary. The treatment considered here involves exposure of the wound to non-invasive low (20-100 kHz) frequency ultrasound energy with periodic real-time digital and near infrared (NIR) monitoring of tissue optical properties related to wound healing parameters. Thus, in vivo acquired diagnostic information provided by an optic sensor will be combined with therapy and used to direct and optimize wound healing treatment. Our ultimate goal is to develop a sterile, patient-friendly patch containing an ultrasound applicator and associated electronic controls that could be directly applied by a patient to the wound. This wearable patch will allow for frequent (daily or even multiple exposures per day) application of the ultrasound therapy without a return of the patient to the clinic and will significantly increase patient compliance with the therapy. In order to accomplish this goal, we will establish the optimal ultrasound exposure parameters that will serve as the basis for the prototype "Band-Aid®"-like wearable ultrasound applicator.
Participating faculty:
Back to Top
Directed Drug Delivery
Surgical procedures that require anastomosis of small lumen structures such as the common bile duct, ureters, and small blood vessels have not benefited from the reliability and ease of surgical stapling. Use of surgical staples for small structures is limited by small structure size and susceptibility to ill effects from scarring at the tissue joint.
We are working to create a new tissue-joining device that will improve on existing staples, including a smaller version for use with small structures. This new staple will be composed of two parts: a rivet with a hollow lumen for flow of bodily fluids and a ring to secure the approximated tissues. Our current research is focusing on the ring. We are developing a biocompatible ring that will degrade and simultaneously deliver drugs.
Improving the speed, accuracy, and effectiveness of procedures, while lowering the morbidity and mortality rates of surgical interventions, is the overall goal of our laboratory. The objective of this project is to develop a new staple for small lumen structures fabricated from an FDA-approved biocompatible, biodegradable polymer that delivers bioactive agents to improve healing. A miniaturized surgical staple that allows delicate structures to be joined safely while maintaining vessel patency will have broad applications in surgery.
Back to Top
Magnetizable Stents for Drug Delivery
The Department of Surgery in collaboration with Dr. Gary Friedman (Electrical and Computer Engineering) and Dr. Kenneth Barbee (School of Biomedical Engineering) are developing a minimally invasive, targeted drug-delivery system that uses magnetic implants and magnetic nanoparticles to administer repeatable, patient-specific dosages of therapeutic agents to specific sites in the human body. The researchers on the team specialize in the fields of magnetism, fluids, cardiovascular biomechanics and medicine, biomaterials, imaging and surgery.
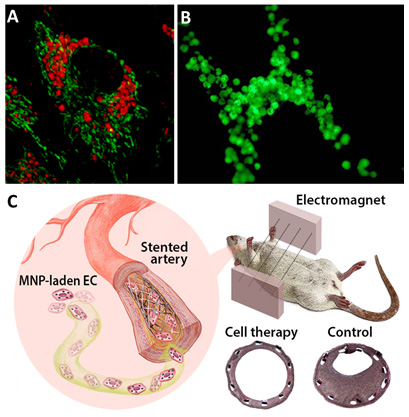 A: Live endothelial cell loaded with magnetic nanoparticles (red). B: Magnetically responsive endothelial cells targeted to a stent via magnetic forces. C: Magnetically guided local delivery of MNP-laden endothelial cells to stented artery mediated by a uniform magnetic field. Magnetically delivered endothelial cells attenuated in-stent stenosis induced by a mechanical injury in carotid artery.
A: Live endothelial cell loaded with magnetic nanoparticles (red). B: Magnetically responsive endothelial cells targeted to a stent via magnetic forces. C: Magnetically guided local delivery of MNP-laden endothelial cells to stented artery mediated by a uniform magnetic field. Magnetically delivered endothelial cells attenuated in-stent stenosis induced by a mechanical injury in carotid artery.
In many cases, currently available drug delivery vehicles (polymer-based particles, liposomes, or hydrogels, for instance) do not have a mechanism for localization that allows delivery of high concentrations of drugs with minimally invasive techniques. This is especially true when repeat dosing is required. Ultrasound-mediated delivery shows promise for regional drug delivery, but lacks the pinpoint accuracy needed for delivery to vascular walls and isolated tumors. The magnetic drug delivery system proposed herein overcomes many of these difficulties and provides a method for concentrating drugs at selected sites in the body with minimal stress on the patient, with much higher dosages than could be accomplished via systemic drug administration, and without the fear of toxicity. The first objective of this project is the prevention of coronary restenosis by adapting this system for use in coronary stents, but if successful, the technology can be expanded to numerous applications ranging from cancer therapy to stem cell delivery. In addition, the incorporation of magnetism into the delivery of the various carriers mentioned above may increase their efficacy and expedite their clinical implementation.
Our central hypothesis is that when implanted in vivo, an endovascular stent plated with small magnetic features or composed of a weakly magnetic alloy can attract injected magnetic nanoparticle (MNP)-laden cells under the application of a modest uniform magnetic field. The external uniform field is used to both magnetize the MNP-laden cells and produce high field gradients on the stent surface. The magnetization effect generates a magnetic force pulling MNP-laden cell therapy to the luminal surface of the stented artery. This procedure is minimally invasive and allows repeat dosing. Furthermore, the therapeutic agent and/or the delivery vehicle can be varied.
Our approach has shown to provide the capability for an accelerated regeneration of vascular tissue via the efficient localization of endothelial cells to stented blood vessels, resulting in fewer restenosis-related complications on the long-term scale. The developed methodology has broad general implications for targeted cell delivery in a number of therapeutic settings using magnetizable steel implants as targeting devices for the ordered deposition of cell-based therapies.
Related Publications
Magnetic Nanoparticle-Mediated Targeting of Cell Therapy Reduces In-Stent Stenosis in Injured Arteries
Boris Polyak, Mikhail Medved, Nina Lazareva, Lindsay Steele, Tirth Patel, Ahmad Rai, Menahem Y. Rotenberg, Kimberly Wasko, Andrew R. Kohut, Richard Sensenig, and Gary Friedman
ACS Nano (September 13, 2016)
"Metabolic and structural integrity of magnetic nanoparticle-loaded primary endothelial cells for targeted cell therapy"
Orynbayeva Z, Sensenig R, Polyak B
Nanomedicine (Lond), 10, 1555-68 (2015)
"Functional behavior and gene expression of magnetic nanoparticle-loaded primary endothelial cells for targeting vascular stents"
Zohra FT, Medved M, Lazareva N, Polyak B
Nanomedicine (Lond), 10, 1391-406 (2015)
"Force dependent internalization of magnetic nanoparticles results in highly loaded endothelial cells for use as potential therapy delivery vectors"
Cristin MacDonald, Kenneth Barbee, and Boris Polyak
Pharmaceutical Research 29(5): 1270-1281 (2012)
"Magnetically responsive paclitaxel-loaded biodegradable nanoparticles for treatment of vascular disease: preparation, characterization and in vitro evaluation of anti-proliferative potential"
Johnson B, Toland B, Chokshi R, Mochalin V, Koutzaki S, and Polyak B
Current Drug Delivery, 1, 7(4), 263-73 (2010)
"Time-varied magnetic field enhances transport of magnetic nanoparticles in viscous gel"
MacDonald C, Friedman G, Alamia J, Barbee K, and Polyak B
Nanomedicine 5, 65-76 (2010)
"Magnetic targeting for site-specific drug delivery: applications and clinical potential"
Boris Polyak and Gary Friedman
Expert Opinion on Drug Delivery, 6(1):53-70 (2009)
"High field gradient targeting of magnetic nanoparticles-loaded endothelial cells to the surfaces of steel stents"
Boris Polyak, Ilia Fishbein, Michael Chorny, Ivan Alferiev, Darryl Williams, Ben Yellen, Gary Friedman, and Robert J. Levy
PNAS, 105(2):698-703 (2008)
Back to Top
Cardiac Tissue Engineering in Magnetically Actuated Scaffolds
One of the major obstacles in engineering thick, complex tissues, such as cardiac muscle, is the need to pre-vascularize the engineered tissue in vitro and create stimulating microenvironment in vivo for efficient construct integration with the host tissue. In addition to molecular signals e.g. growth factors, mechanical cues are also required for generation of functional cell constructs. Mechanical cues are particularly relevant to cardiac muscle and vasculogenesis, because mechanical stresses activate mechano-sensitive receptors initiating pathways that lead to development of functional tissue. The Department of Surgery in collaboration with Prof. Smadar Cohen (Biotechnology Engineering, Ben-Gurion University, Israel) are developing magnetically actuated alginate-based scaffolds for non-invasive (remote) application of mechanical cues to artificially-generated cell constructs in vitro and after their implantation in vivo.
Our team has demonstrated the feasibility of ‘magneto-mechanical’ stimulation in 3D cultures of endothelial and cardiac cells. Using atomic force microscopy, we recently found that magnetic scaffolds exposed to a time-varying uniform magnetic field undergo a reversible shape deformation imparting a mechanical force on cells on the order of 1pN. These results validate the possibility of generating forces due to the alternating pattern of scaffold wall alignment and relaxation that correlate with the reported threshold for inducing cellular mechanotransduction. The uniqueness and novelty of our tissue engineered scaffolds and remote cell stimulation approach is in the ability to generate mechanical cues using time-varying uniform magnetic fields. Generation of uniform magnetic fields across large animals or even humans is a relatively easy and scalable task, as opposed to situations where gradient fields are used for the same purpose. This distinction makes our approach highly innovative and suitable for the future clinical translation.
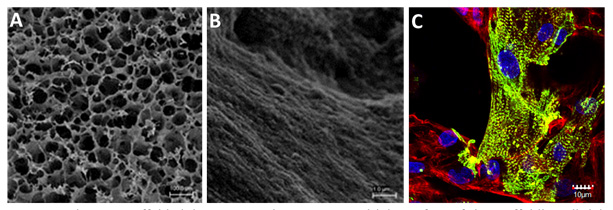 Magnetic alginate scaffolds (A) macro-scale structure and (B) surface of the scaffold's wall. (C) Functionally organized neonatal cardiomyocytes stimulated by AC magnetic field. Massive striation (in green) indicates on a functionally organized sarcomeric α-actinin. Non-myocyte cells providing a supportive layer to cardiomyocytes are stained in red.
Magnetic alginate scaffolds (A) macro-scale structure and (B) surface of the scaffold's wall. (C) Functionally organized neonatal cardiomyocytes stimulated by AC magnetic field. Massive striation (in green) indicates on a functionally organized sarcomeric α-actinin. Non-myocyte cells providing a supportive layer to cardiomyocytes are stained in red.
Related Publications
"Magnetically actuated tissue engineered scaffold: insights into mechanism of physical stimulation"
Sapir-Lekhovitser Y, Rotenberg MY, Jopp J, Friedman G, Polyak B, Cohen S
Nanoscale, 8, 3386-99 (2016)
"Cardiac Tissue Engineering in Magnetically Actuated Scaffolds"
Yulia Sapir, Boris Polyak and Smadar Cohen
Nanotechnology Jan 10;25(1) (2014)
"Magnetic nanoparticle-based approaches to locally target therapy and enhance tissue regeneration in vivo"
Richard Sensenig, Yulia Sapir, Cristin MacDonald, Smadar Cohen, and Boris Polyak
Nanomedicine 7(9):1425-42 (2012)
"The promotion of in vitro vessel-like organization of endothelial cells in magnetically responsive alginate scaffolds"
Sapir Yulia, Cohen Smadar, Friedman Gary and Polyak Boris
Biomaterials 33(16): 4100-4109 (2012)
Back to Top
Magnetic Cell Labeling
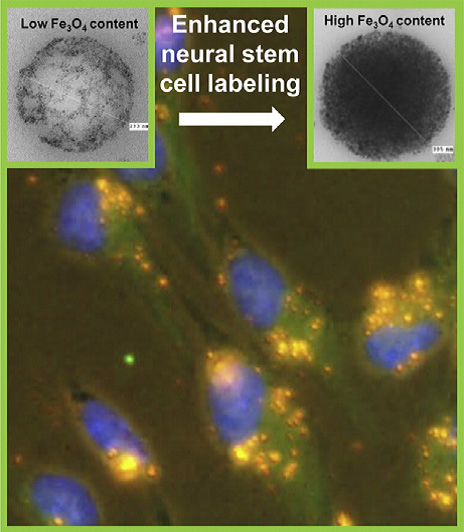
Safe and efficient delivery of therapeutic cells to sites of injury/disease in the central nervous system is a key goal for the translation of cell transplantation therapies. Recently, magnetic cell localization strategies have emerged as a promising and safe strategy for the targeted delivery of magnetic nanoparticles (MNP) labeled stem cells to sites of pathology. For neuroregenerative applications, this approach is limited by the lack of available neurocompatible MNPs and low levels of cell labeling achieved in neural stem/precursor populations, representing a major translational barrier.
The Department of Surgery in collaboration with Prof. Divya Maitreyi Chari (Neural Tissue Engineering, Keele University, UK) have recently developed a facile approach using high magnetite content, self-sedimenting polymeric MNP to achieve high efficiency labeling (≥ 90%) of primary neural stem cells, a ‘hard-to-label’, transplant population of high clinical relevance. The developed protocols resulted in high safety with respect to key stem cell regenerative parameters. Critically, labeled cells were effectively localized in a flow system by magnetic force highlighting the clinical translational potential of the methods described.
Related Publications
"Endocytotic potential governs magnetic particle loading in dividing neural cells: studying modes of particle inheritance"
Tickle JA, Jenkins SI, Polyak B, Pickard MR, Chari DM
Nanomedicine (Lond), 11, 345-58 (2016)
"Increasing magnetite contents of polymeric magnetic particles dramatically improves labeling of neural stem cell transplant populations"
Adams CF, Rai A, Sneddon G, Yiu HH, Polyak B, and Chari DM
Nanomedicine: Nanotechnology, Biology and Medicine 11, 19-29 (2015)
Back to Top
Tracking Inflammation Associated With Brain Disorders
Accumulating evidence suggests that activated immune cells may serve as a biomarker of epileptic foci. The Department of Surgery in collaboration with Dr. Sara Eyal (School of Pharmacy, The Hebrew University, Israel), Dr. Dana Ekstein (Neurology, Hadassah Medical Center, Israel), Prof. Shlomo Magdassi (Casali Center, Institute of Chemistry, The Hebrew University of Jerusalem, Israel) and Dr. Timothy Roberts (Radiology, The Children’s Hospital of Philadelphia) recently demonstrated that systemically injected biocompatible nanoparticles detectable by both optical and magnetic resonance imaging (MRI) techniques identify activated myeloid cells in epileptogenic brain tissue. We demonstrated that intravenously-administered nanoparticles can target myeloid cells in epileptogenic brain tissue. This system may contribute to pre-surgical and intra-surgical localization of epileptic foci and assist in detecting immune system involvement in epilepsy.
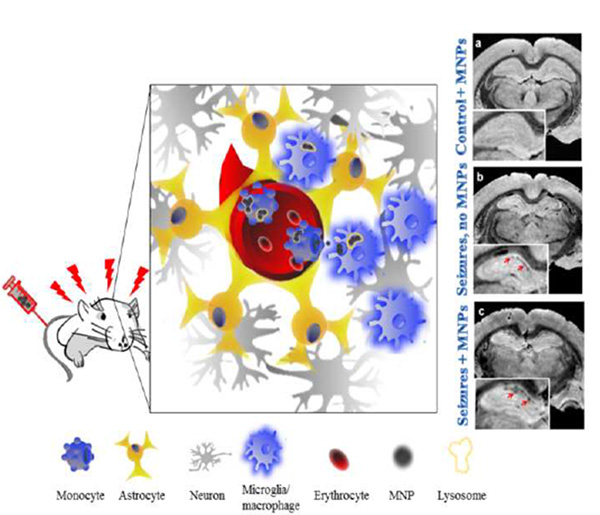 Bi-functional fluorescent and magnetic nanoparticles (MNPs) were used to image epileptogenic tissue in the rat brain. The MNPs were injected intravenously to rats with chronic spontaneous seizures. MNPs selectively accumulated within myeloid cells in the hippocampus, in association with inflammation. The MNP signal was detectable by immunohistochemistry and by MRI. This system can contribute to presurgical and intra-surgical localization of epileptic foci, and assist in detecting immune system involvement in epilepsy.
Bi-functional fluorescent and magnetic nanoparticles (MNPs) were used to image epileptogenic tissue in the rat brain. The MNPs were injected intravenously to rats with chronic spontaneous seizures. MNPs selectively accumulated within myeloid cells in the hippocampus, in association with inflammation. The MNP signal was detectable by immunohistochemistry and by MRI. This system can contribute to presurgical and intra-surgical localization of epileptic foci, and assist in detecting immune system involvement in epilepsy.
Related Publication
"Tracking inflammation in the epileptic rat brain by bi-functional nanoparticles"
Portnoy, Emma; Boris Polyak, Inbar, Dorrit, Kenan, Gilad, Rai, Ahmad, Wehrli, Suzanne, Roberts, Timothy, Bishara, Ameer, Mann, Aniv, Shmuel, Miriam, Rozovsky, Katya, Itzhak, Gal, Ben Hur, Magdassi, Shlomo, Ekstein, Dana, Eyal, Sara
Nanomedicine: Nanotechnology, Biology and Medicine 12, 1335–1345 (2016)
Back to Top
Detection of Colon Cancer by Tumor-Specific Nanoparticles
Ulcerative colitis (UC) is associated with a well-recognized risk for development of large bowel adenocarcinoma, which is often linked to antecedent or synchronous epithelial dysplasia. Regardless of its shortcomings, periodic endoscopic surveillance with multiple biopsies is now recommended to identify individuals with dysplasia complicating their ulcerative colitis. However, a large number of biopsies is necessary to ensure 90% confidence in detection of dysplasia and cancer if present. Unfortunately, compliance with these recommendations widely varies among practitioners. Emerging molecular imaging utilizing near infra-red (NIR) fluorescent nanoparticles (NPs) may help us to improve neoplasm detection sensitivity and enable reliably identify persons with ulcerative colitis at significant risk for imminent neoplasia. We hypothesize that colon cancer specific nanoparticles can target areas of dysplasia in induced colon cancer rat model and enable real-time, specific and sensitive detection of pathological tissue at various stages of cancer development. The benefit of this research is to more accurately diagnose colon cancer in an animal model that can be translated in future to a more quick and confident diagnosis of colitis-associated dysplasia in humans. The long term goal of this research is to exploit NIR NP technology and develop a new diagnostic and potentially therapeutic modality for patients with colitis-associated dysplasia and de novo colon cancer. Physicians would be able to identify dysplastic areas early and decrease the incidence of colorectal cancer in this patient population.
* Physician's practice is independent of Drexel University College of Medicine.
Back to Top