Research
Melanoma is the deadliest form of skin cancer; as patients presenting with metastatic disease have a five-year survival of only 2-16%. However, early clinical intervention prior to metastatic dissemination yields ten-year survival rates of ~92%. Our goal is to better understand mechanisms that promote melanoma metastasis and the circumstances governing targeted inhibitor efficacy.
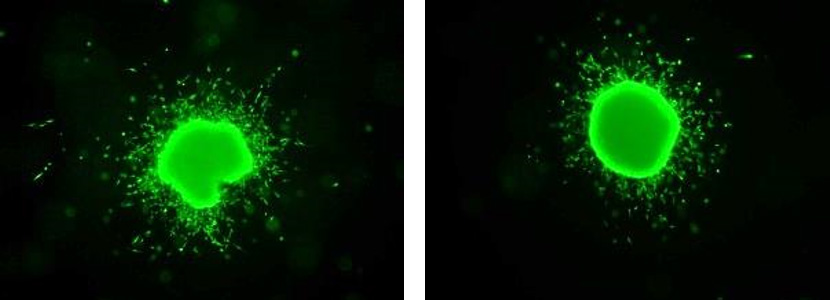
Melanoma spheroid invading into collagen.
Through transcriptomic analysis, we have identified cell adhesion molecule 1 (CADM1) as a suppressor of metastatic traits in human melanoma. CADM1 levels are inversely associated with tumor stage and high CADM1 is correlated with better prognosis. We found that CADM1 inhibits melanoma disease progression by suppressing invasiveness and potently induces non-adherent cell death – two properties critical to metastatic dissemination. Utilizing multi-disciplinary approaches we are currently investigating the mechanisms by which CADM1 is regulated and how it signals. Additionally, we are interested in assaying the role CADM1 plays in melanoma immune surveillance and determining if CADM1 expression could be considered a diagnostic marker for immunotherapy efficacy.

CADM1 expressing melanoma cells.

CADM1 expressing melanoma cells.
A secondary focus of the laboratory is defining novel therapeutics, resistance mechanisms, and second-line therapy options for patients suffering from mutant BRAF driven cancers. BRAF is a serine/threonine kinase in the in the RAS-RAF-MEK-ERK mitogenic pathway. Activating mutations in this pathway lead to many human malignancies. Notably, BRAF is altered in ~50% of melanomas, 60% of thyroid carcinomas, and ~10% of colorectal cancers. The most prevalent mutation of BRAF occurs at position 600 where valine is substituted for either glutamic acid or lysine (BRAFV600E/K). BRAFV600E/K mutant selective inhibitors have been developed and are currently in clinical use, however these drugs are only efficacious in certain settings and patients that do benefits from these agents often undergo drug resistance and eventual relapse. We aim to study a "next-generation" mutant BRAF inhibitor, PLX8394, currently in clinical trials. Our recent suggest that PLX8394 treatment uniquely breaks RAF level dimerization. This is an important attribute that should be further explored as existing FDA-approved RAF inhibitors are ineffective against mutant BRAF cancers known to have high RAF dimerization. Through in vitro, in vivo, and ex vivo experimentation we will assay the effectiveness of PLX8394 in a panel of dimerization dependent non-canonical mutant BRAF melanoma, and mutant BRAF colorectal and thyroid cancers.

Overview of resistance mechanisms to RAF inhibitors in mutant BRAF melanoma.
Back to Top