'Chemical Net' Could Be Key to Capturing Pure Hydrogen

- Drexel Environmental Collaboratory Releases Cross-Sector Findings on Severe Weather Recovery Challenges
- Drexel Launches the Manuel Stamatakis Center for Alternative Investments at the LeBow College of Business
- How and When Could AI Be Used in Emergency Medicine?
- Faculty Highlights: Recent Awards and Grants
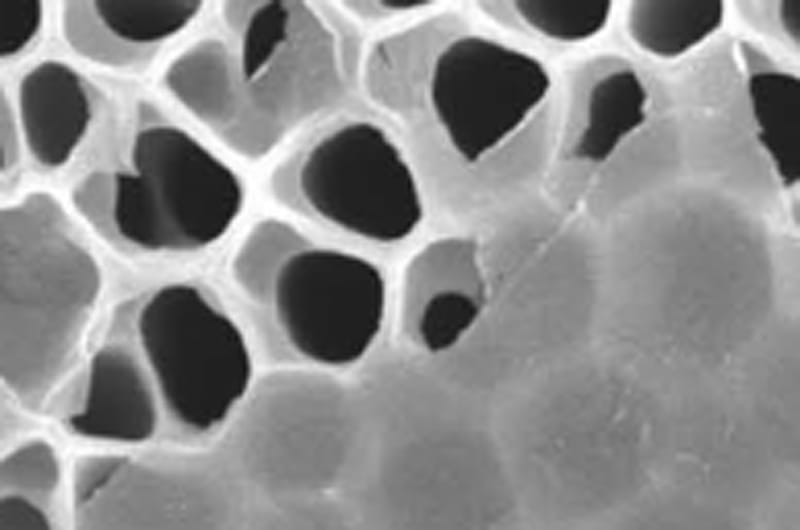
A team of international researchers, including Drexel's Yury Gogotsi, PhD, are studying MXene for use in gas separation.
Hydrogen is one of the most abundant elements on Earth and an exceptionally clean fuel source. While it is making its way into the fuel cells of electric cars, busses and heavy equipment, its widespread use is hampered by the expensive gas-separation process required to produce pure hydrogen. But that process could soon become more efficient and cost-effective thanks to a discovery by an international team of researchers, led in the U.S. by Drexel University. The group has uncovered exceptionally efficient gas separation properties in a nanomaterial called MXene that could be incorporated into the membranes used to purify hydrogen.
While hydrogen is present in a wide variety of molecules and materials in nature — water, a combination of hydrogen and oxygen, foremost among them — it does not naturally exist in its pure elemental form — that is, hydrogen on its own, on Earth. To separate hydrogen from the other elements to which it commonly bonds, it requires introducing an electric current to excite and split apart the atoms in water molecules, or filtering a gaseous mixture containing hydrogen, through a membrane to separate the hydrogen from carbon dioxide or hydrocarbons.
The process of gas separation via membrane is the more effective and affordable option, so in recent years researchers have been ramping up efforts to develop membranes that can thoroughly and quickly filter out hydrogen.

A study recently published in the journal Nature Communications, indicates that using MXene material in gas-separation membranes could be the most efficient way to purify hydrogen gas. The research, led by Haihui Wang, PhD, a professor from South China University of Technology and Yury Gogotsi, PhD, Distinguished University and Bach professor in Drexel’s College of Engineering, in the Department of Materials Science and Engineering, shows that the nanomaterial’s two-dimensional structure enables it to selectively reject large gas molecules, while letting hydrogen slip between the layers.
“In this report we show how exfoliated two-dimensional MXene nanosheets can be used as building blocks to construct laminated membranes for gas separation for the first time,” Gogotsi said. “We demonstrated this using model systems of hydrogen and carbon dioxide.”
Working in collaboration with researchers from South China University of Technology and Jilin University, in China, and Leibniz University of Hannover, in Germany, the Drexel team reported that membranes created using MXene nanosheets outperform the top-of-the-line membrane materials currently in use — both in permeability and selectivity.
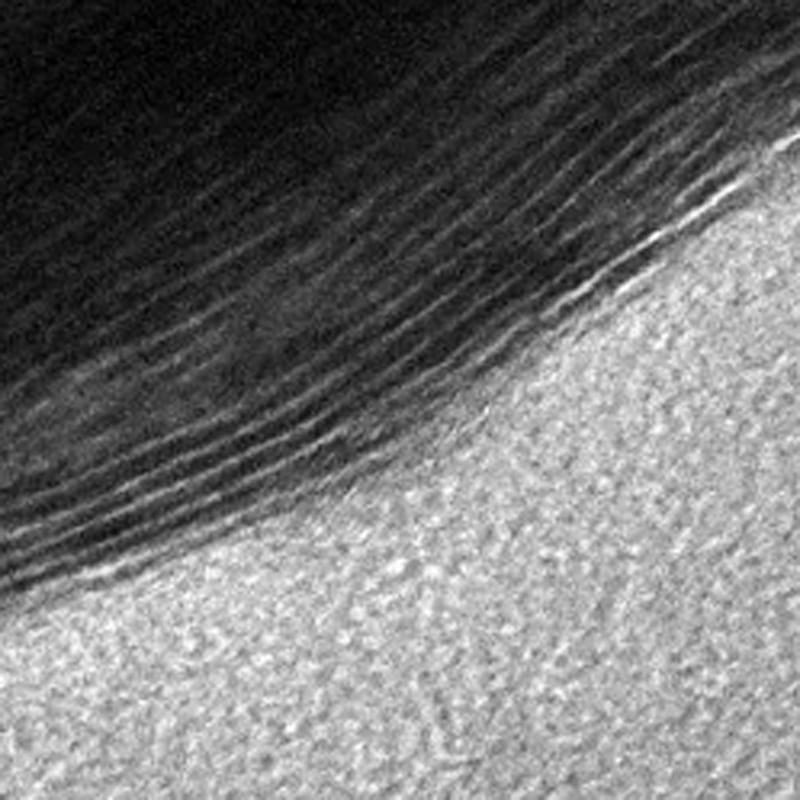
Many different kinds of membranes are currently in use throughout the energy industry, for example for purifying coolant water before it is released, and for refining natural gas before it is distributed for use. Gas separation facilities also use them to retrieve nitrogen and oxygen from the atmosphere. This study opens the door for an expanded use of membrane technology, with the possibility of tailoring the filtration devices to sift out a large number of gaseous molecules.
MXene’s advantage over materials currently being used and developed for gas separation is that both its permeability and filtration selectivity are tied to its structure and chemical composition. By contrast, other membrane materials, such as graphene and zeolite, do their filtering only by physically trapping — or sieving — molecules in tiny grids and channels, like a net.
MXene's special filtration properties exist because they are created by chemically etching out layers from a solid piece of material, called a MAX phase. This process forms a structure that is more like a sponge, with slit pores of various sizes. Gogotsi’s Nanomaterials Research Group, which has been working with MXenes since 2011, can predetermine the size of the channels by using different types of MAX phases and etching them with different chemicals.
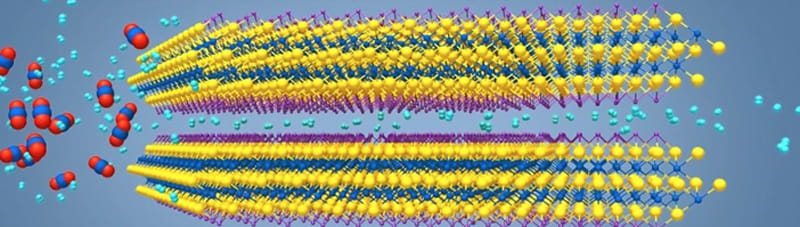
The channels themselves can be created in a way that makes them chemically active, so they are able to attract — or adsorb — certain molecules as they pass through. Thus, a MXene membrane functions more like a magnetic net and it can be designed to trap a wide variety of chemical species as they pass through.
“This is one of the key advantages of MXenes,” Gogotsi said. “We have dozens of MXenes available which can be tuned to provide selectivity to different gasses. We used titanium carbide MXene in this study, but there are at least two dozen other MXenes already available, and more are expected to be studied in the next couple of years — which means it could be developed for a number of different gas separation applications.”
The versatile two-dimensional material, which was discovered at Drexel in 2011, has already shown its ability to improve efficiency of electric storage devices, stave off electromagnetic interference and even purify water. Studying its gas separation properties was the next logical step, according to Gogotsi.
“Our work on water filtration, the sieving of ions and molecules, and supercapacitors, which also involves ion sieving, suggested that gas molecules may also be sieved using MXene membranes with atomically thin channels between the MXene sheets,” he said. “However, we were lacking experience in the gas separation field. This research would not have been possible without our Chinese collaborators, who provided the experience needed to achieve the goal and demonstrated that MXene membranes can efficiently separate gas mixtures.”
In order for MXene to make its way into industrial membranes, Gogotsi’s group will continue to improve its durability, chemical and temperature stability and reduce the cost of production.
Read the full paper here: https://www.nature.com/articles/s41467-017-02529-6
Drexel News is produced by
University Marketing and Communications.