MXenes combine the rare combination of metallic conductivity and hydrophilicity with two-dimensional morphology, which make them distinctly different from other classes of energy storage materials such as carbons and metal oxides. Highly accessible and reversible nature of transition metal redox reactions is a unique aspect, bringing special focus in exploring MXenes for energy storage applications. The versatility of MXene materials opens new avenues for resolving future energy demands.
MXene as a Charge Host
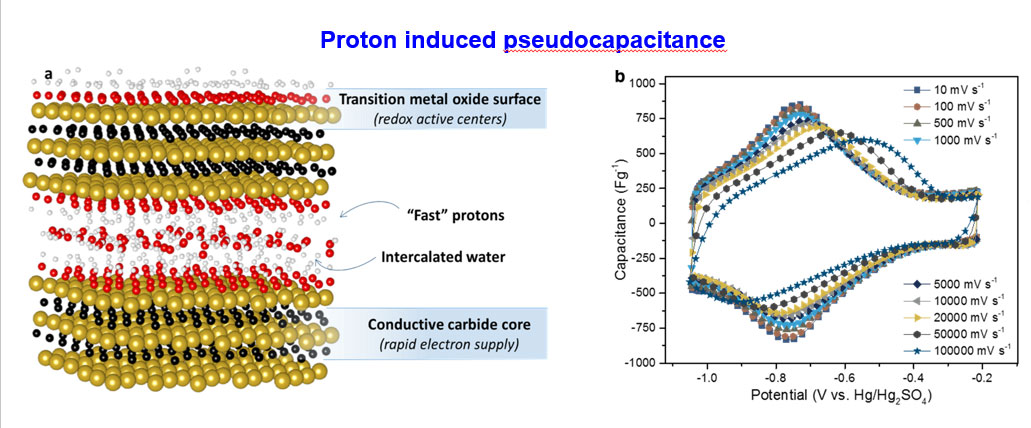
Is it possible to charge your cell phone in a few seconds? The answer is yes, and recent studies demonstrated that highly conductive MXene electrodes enable future demand of fast charging technology. MXene galleries provide super-fast lanes for ions travel unlike the narrow paths of porous carbon electrodes. Thanks to the swift redox reactions at the transition metal surface, offering an order of magnitude higher capacities over carbon materials at much faster rates.
Related publications:
- B. Anasori, M. R. Lukatskaya, Y. Gogotsi, 2D metal carbides and nitrides (MXenes) for energy storage, Nature Reviews Materials, 2, 16098 (2017)
- M. R. Lukatskaya, S. Kota, Z. Lin, M.-Q. Zhao, N. Shpigel, M. D. Levi, J. Halim, P.-L. Taberna, M. W. Barsoum, P. Simon, Y. Gogotsi, Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides, Nature Energy, 2, 17105 (2017)
- X. Wang, T.S. Mathis, K. Li, Z. Lin, L. Vlcek, T. Torita, N.C. Osti, C. Hatter, P. Urbankowski, A. Sarycheva, M. Tyagi, E. Mamontov, P. Simon, Y. Gogotsi, Influences from solvents on charge storage in titanium carbide MXenes Nature Energy, 4, 241 (2019)
- Y. Xia, T. Mathis, M.-Q. Zhao, B. Anasori, A. Dang, Z. Zhou, H. Cho, Y. Gogotsi, S. Yang, Thickness Independent Capacitance of Vertically Aligned Liquid Crystalline MXenes, Nature, 557, 409–412 (2018)
MXenes for Storing Multi-valent Ions
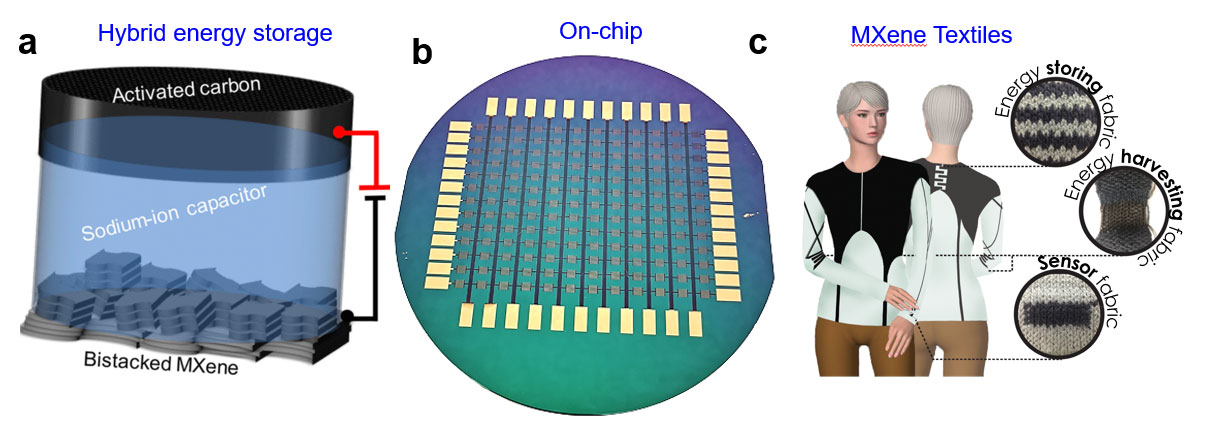
Lithium-ion batteries are already a dominant technology in portable and flexible electronics industry. However, the cost and democratic reserves of Li resources raise the concerns for the future electrification and large-scale energy storage. Going “beyond Li-ion technology” needs robust electrodes for reversible (de) intercalation of metal-ions. As MXenes offer spontaneous intercalation of a variety of cations (Na+, K+, Mg+2, Zn+2, Ca+2 and Al+3), they could be the potential alternatives to conventional carbon and metal oxide materials for the design of hybrid metal capacitors and multi-valent batteries.
Can we downscale the energy storage devices for powering of microelectronics?
The current trend of emerging Internet of Things (IOT) and miniaturization of electronics demand for developing small scale energy storage devices. Scalable manufacturing of On-chip MXene devices can offer compatible integration with micro robotics, sensors and bio-medical implants. Additionally, transparent conducting behavior and color changing properties make MXenes suitable for developing transparent and electrochromic energy storage devices. Furthermore, MXenes can be used for flexible and wearable applications where MXene composites can combine the roles of energy storage, harvesting and sensing capabilities.
Related publications:
- N. Kurra, M. Alhabeb, K. Maleski, C.-H. Wang, H. Alshareef, Y. Gogotsi, Bilayer Titanium Carbide (MXene) Anodes for Hybrid Sodium Ion Capacitors, ACS Energy Letters, 3, 2094−2100 (2018)
- Q. Jiang, N. Kurra, K. Maleski, Y. Lei, H. Liang, Y. Zhang, Y. Gogotsi, H. N. Alshareef, On-chip MXene Microsupercapacitors for AC-line Filtering Applications, Advanced Energy Materials, 9 (26) 1901061 (2019)
- A. S. Levitt, M. Alhabeb, C. B. Hatter, A. Sarycheva, G. Dion, Y. Gogotsi, Electrospun MXene/Carbon Nanofibers as Supercapacitor Electrodes, Journal of Materials Chemistry A,7, 269 – 277 (2019)
Versatility of MXenes in Energy Storage Devices
Aside from acting as a main electrochemically active component in a variety of energy storage devices, functional MXene surface can play multiple roles such as binder, conductive additive, and current collectors.
MXene films and coatings offer metallic conductivity and can potentially replace standard metal based current collectors (copper, aluminum and gold) in the energy storage devices. High conductivity and strong binding properties of MXene particles, enable them to be better alternatives than typically employed polymeric binders (Polyvinylidene fluoride, Polytetrafluoroethylene) and conductive additives (carbon black) in conventional energy storage devices.
More information and related information
- C. J. Zhang, S.-H. Park, A. Seral‐Ascaso, S. Barwich, N. McEvoy, C. S. Boland, J. N. Coleman, Y. Gogotsi, V. Nicolosi, High Capacity Silicon Anodes Enabled by MXene Viscous Aqueous Ink, Nature Communications, 10, 849 (2019)
- L. Yu, L. Hu, B. Anasori, Y.-T. Liu, Q. Zhu, P. Zhang, Y. Gogotsi, B. Xu, MXene-Bonded Activated Carbon as a Flexible Electrode for High-Performance Supercapacitors, ACS Energy Letters, 3, 1597-1603 (2018)
MXenes for Energy Conversion
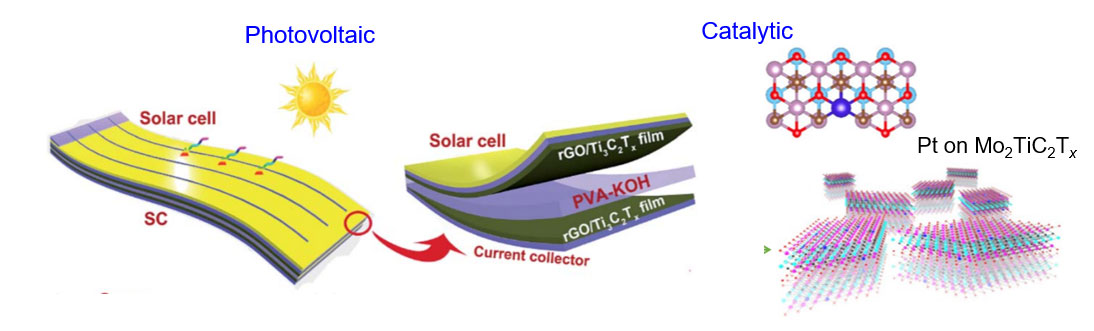
MXene coatings have shown significant improvement in the efficiency of perovskite solar cells by improving charge transport pathways. Due to both energy storage and harvesting capabilities of MXenes, it is possible to design integrated devices where MXenes play dual roles in energy storage and conversion applications.
Unlocking the Catalytic Properties of MXenes
Can MXenes be used for generating green energy? Yes, inherent transitional metal defects is the key for catalytic activity of MXenes towards nitrogen fixation and water splitting applications, which enable future hydrogen and oxygen fuel cell technologies. Since MXenes offer catalytic sites throughout the basal planes, their catalytic properties are different and superior when compared to 2D metal sulfides/selenides with only edge sites that are catalytically active.
Related publications:
- J. Zhang, Y. Zhao, X. Guo, C. Chen, C.-L. Dong, R.-S. Liu, C.-P. Han, Y. Li, Y. Gogotsi, G. Wang, Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction, Nature Catalysis, 1, 985–992 (2018)
- S. Xu, G. Wei, J. Li, X. Pan, W. Han, Y. Gogotsi, Flexible MXene-graphene electrodes with high volumetric capacitance for integrated co-cathode energy conversion/storage devices, J. Mater. Chem. A, 5, 17442 – 17451 (2017)
- L. Yang, C. Dall’Agnese, Y. Dall’Agnese, G. Chen, Y. Gao, Y. Sanehira, A. K. Jena, X.-F. Wang, Y. Gogotsi, T. Miyasaka, Surface-Modified Metallic Ti3C2Tx MXene as Electron Transport Layer for Planar Heterojunction Perovskite Solar Cells, Advanced Functional Materials, 1905694