Mobilized: Drexel Researchers Face Down the Coronavirus
Fighting a global pandemic requires innovation and urgency, and Drexel’s researchers have heeded the call. Vaccine adjuvants, infection blockers and protein targeting to inactivate the virus are among the 17 COVID-19 projects currently supported by the $100,000 Rapid Response Research and Development Fund, created by University trustees. More than half the awarded projects involve College of Medicine faculty.
“When the pandemic started, Drexel research was shut down except for essential COVID-19 investigations,” says Irwin Chaiken, PhD, professor of biochemistry and molecular biology. “Investigators considered the problem, the urgency to respond and what they could do to help.”
Stopping the Virus in Its Tracks
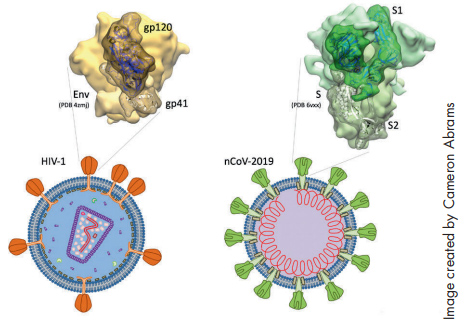
Chaiken drew from his ongoing research in HIV when he developed the concept for his study “Irreversible Inactivation of SARS-CoV-2 by Spike Protein Targeting.” Chaiken and his team recognized right away that the primary protein on the surface of the SARS-CoV-2 virus had properties that were similar to the spike protein in HIV. The idea was to use the same technology they had developed for HIV to target the new virus.
While the spike protein has been widely established as a target for treating SARS-CoV-2, it’s Chaiken’s use of lectin-DLIs (dual action lytic inhibitors) that makes his work particularly novel and could lead to the development of a therapy that would actually render the virus inactive. The team has tested the concept on a pseudovirus with positive results.
“We found that in fact the lectin-DLIs did inhibit infection by SARS-CoV-1, and preliminary data suggests that it will work on SARS-CoV-2,” Chaiken says. “It’s worth noting that the spike protein for SARS is also crucial for the development of vaccines.”
A Vaccine That Goes the Distance
To that end, Chaiken has been collaborating with another project team, led by infectious disease experts Elias El Haddad, PhD, professor of medicine, and Michele Kutzler, PhD, associate professor of medicine, and microbiology and immunology. They are working to develop a vaccine using both an antigen and an adjuvant.
“We know that control of this pandemic will be challenging without a vaccine,” Kutzler says. “We saw an opportunity to pivot our research and develop a vaccine for the prevention of this disease.”
In particular, they wanted to test out the adjuvant molecule Haddad had developed, adenosine deaminase-1 (ADA-1), an enzyme critical for maintenance and function of the immune system, which has been shown to boost the activity of T cells. They are partnering with David Weiner, PhD, of the Wistar Institute on an immunoadjuvant system for a potential COVID vaccine. They are doing preclinical collaboration with the Weiner Lab on the ADA-1 adjuvant and a DNA vaccine encoding SARS-CoV2 S1 glycoprotein. The goal is to evaluate the immunogenicity of the vaccine and see if its effects would be more potent and longer lasting with the addition of ADA-1.
“The knowledge that immune function declines with age and that there is greater risk of severe COVID-19 in older adults and immune-compromised individuals leading to poor vaccine responses leads us to believe that development of effective adjuvants is key,” Kutzler says. “In addition, we’re looking for improved immune memory and durability in this next generation of vaccine formulations.”
The team is hoping to complete the preclinical work by the end of the year and begin clinical trials early next year.
Blocking Infection at the Gate
At the time of the initial outbreak, Simon Cocklin, PhD, an associate professor, and Adel Rashad, PhD, research assistant professor, both in the Department of Biochemistry and Molecular Biology, were collaborating with a laboratory at Shandong University in Jinan, China, that was directly impacted.
Better Protection by Design
Rapid Response grants went to several proposals using technology and design to protect and support patients, health care workers and special populations. College of Medicine Dean Charles B. Cairns, MD, has developed a COVID-19 information-sharing mobile app called the Drexel Health Tracker. With the collective health of the Drexel community in mind, the app is part of the school’s evidence-based public health measures, and is being used to monitor individual health through self-reporting, in concert with testing protocols. Funding was also awarded to a collaboration by Dean Cairns and Westphal College of Media Arts & Design professor Genevieve Dion, director of the Center for Functional Fabrics, to create protective face masks for health care workers and the general public. In the sphere of public and mental health, Psychiatry Department faculty Drs. David Bennett and Barbara Schindler are leading the project “Implementation of an Online Peer Support Community to Assist Women with Substance Abuse Disorder during the COVID-19 Pandemic: A Pilot Study,” with collaborators in the College of Computing and Informatics. In addition, Michael Weingarten, MD, MBA, professor of surgery, is working with a team from the College of Nursing and Health Professions on the study “Chronicling the Impact of the COVID-19 Pandemic on Physical and Mental Health and Telehealth Care Delivery: Perspectives from Providers and Older Adults.”
“Our partners in China were concerned about CoV-2 and asked if we would help on the biology side of testing new antivirals for the virus,” Cocklin says. Cocklin and Rashad’s study, “Macrocyclic and Small Molecule Inhibitors of SARS-CoV-2 Entry,” is focused on developing molecules that could stop the virus and initial infection at the level of cellular entry.
“Based on the nature of the SARS-CoV-2 glycoproteins, we decided to go with macrocyclic compounds, which are effectively large small molecules so they have more interaction sites and can be optimized to incredible potencies,” Cocklin says.
So far, the team has completed computational screening of almost 13,000 macrocycles against the glycoproteins. “After prioritizing and purchasing a smaller subset of these compounds, we found two that we demonstrated experimentally to interact with the SARS-CoV-2 spike glycoprotein complex,” Cocklin says. “Testing of these compounds in an antiviral pseudovirus screening assay demonstrated that they interfered with the virus entry into cells, but not very potently. Therefore, we subsequently identified and computationally assessed some close variations of these macrocyclic compounds that were commercially available. Purchase and testing of these new compounds allowed us to discover a novel, specific macrocyclic compound inhibitor of SARS-CoV-2 entry that works 20-fold better than our original hits. We are currently seeking funds to continue to identify and optimize this promising class of anti-SARS-CoV-2 molecules.
“These types of inhibitory molecules could be given to at-risk populations while we are waiting for an effective vaccine. They could have widespread benefits for those already infected with SARS-CoV-2, and those awaiting administration of the approved vaccine,” Cocklin says. “They could also provide a treatment option for those who will be ineligible for COVID-19 vaccination due to age, being immunocompromised, or other preexisting medical conditions.”
Cellular Pathways of Protection
Sonia Navas-Martin, PhD, and Julio Martin-Garcia, PhD, associate professors of microbiology and immunology, are looking into the healing properties of macrophages, or white blood cells used as an innate first-line immune defense, to eliminate the virus and how these “peacekeeping cells” might be deployed as therapeutic intervention against SARS-CoV-2.
“Inflammation is a double-edged sword and although it is required for resolution from infections, it can be detrimental and contribute to fatal outcomes,” Navas-Martin says. “Based on my previous expertise on coronaviruses and my long-term collaboration with Dr. Martin-Garcia studying macrophage response to RNA virus infection, we first decided to focus on an important gap of knowledge in coronavirus’ mechanisms of disease.” The team is investigating macrophage responses to SARS-CoV-2 infection, specifically how the virus inhibits macrophages and how macrophage activations might change as the cells age. With preliminary data in hand, they can start to address some of these questions.
“Our SARS-CoV-2 research moves forward in several directions, but clearly we want to tackle cell pathways to control inflammation. The main question is how the virus kills in some people, particularly the elderly or those with comorbidities. Perhaps the right question to ask is how our immune system contributes to those fatal outcomes. At the end, it is a balance between the virus and the host, and identifying individual cells’ responses is key to successfully winning this pandemic battle.” Navas-Martin has been studying coronaviruses since 1999, and she has been fascinated by their unique properties ever since. The biggest challenge in confronting COVID-19 will be ongoing integration of basic and translational research. In a sense, she says, the virus has been a reminder from Mother Nature that we’re all in this together.
“Solutions to this pandemic — antivirals, vaccines, etc.— will be identified through scientific collaboration rather than wild competition,” she says. “It is a lesson that we all have to learn.”
Back to Top