Research Overview and Interests
The Qiang Laboratory concentrates on using human induced pluripotent stem cell (hiPSC) based technologies to establish in vitro (distinct phenotypical CNS neuronal cultures) and ex vivo (3d forebrain and midbrain organoids, aka “mini-brains”) cellular and tissue models to study various neurodegenerative disorders including Tauopathies (including Alzheimer’s Disease, Parkinson’s disease and Frontotemporal Dementia), Hereditary Spastic Paraplegia (HSP) and Gulf War Illness (GWI). The ultimate goal of our mission is to elucidate the etiology and causative mechanisms underlie those disorders and to uncover novel molecular targets for treatment therapies.

Representative images of human forebrain organoids used in modeling GWI.
Lab Personnel
- Emanuela Piermarini, Research Associate
- Larisa Ibric, Lab Manager
- Xiaohuan (Beanie) Sun, PhD Student
- Neha Mohan, PhD Student
- Simeon Kofman, BS/MS Student
- Skandha Ramakrishnan, MS Student
Research Projects
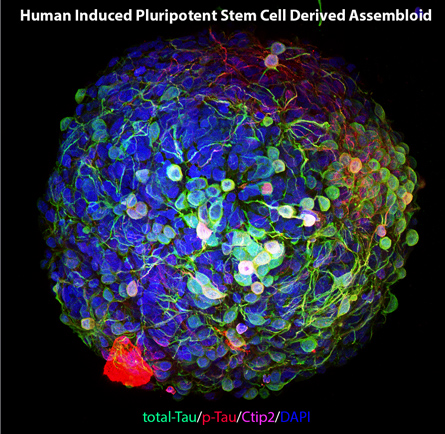
Human induced pluripotent stem cell derived assembloid.
The etiology investigation of HSP.
We are delving into the mechanisms that underlie the etiology of SPG4 (spastin) based HSP. Elucidating the pathological impact of both loss-of-function of spastin (haploinsufficiency) and gain-of-toxicity of mutant spastin provides essential insights into developing target specific therapeutic strategies for HSP. Isogenic hiPSC lines (via CRISPR-Cas9) containing distinct types of spastin mutations, as well as the spastin knockout line, were generated for developing various types of CNS neurons and forebrain organoids to model the disease. Transgenic mouse models were also established in my lab for complementary studies.
Microtubule related deficits in tauopathies.
Our work challenges a significant body of literature on MT-stabilizing drugs as therapy for tauopathies and hence could dramatically alter the trajectory of the field based on our recent findings in tau-KD neurons. We posit both loss-of-function and gain-of-function mechanisms are implicated in developing various pathological phenotypes in tauopathic neurons. Isogenic hiPSC lines (via CRISPR-Cas9) containing distinct types of tau mutations were established in the lab.
CNS susceptibility of GWI and the role of neuroinflammation GWI development.
We obtained hiPSC lines from GW veterans with and without GWI. Human neuronal cultures, as well as forebrain organoids, were generated from those hiPSCs to be used as CNS models for identifying neuronal specific vulnerabilities. They are also used to screen for agents that may alleviate some of symptom related cellular phenotypes. We are also interested in astocytic and microglial contribution to the disease development. We are in the process of establishing those cells types in the lab.
Multidisciplinary approaches applied in Qiang Lab
- hiPSC derived cell and tissue modeling
- ICC/IHC
- Single-cell RNAseq
- Mass-Spec/Proteomics
- Live-cell imaging
- Mouse behavioral analyses
- RNAi/ASO and virus based gene therapies
Selected Grants Funded
- Pennsylvania Department of Health Research Grant (SAP #4100089350) – Gene Therapy via Spastin Overexpression to Promote Axon Regrowth for SCI Repair
- Spastic Paraplegia Foundation Research Grant – Elucidate impaired autophagy as one of the major contributors to SPG4-based Hereditary Spastic Paraplegia
- R01NS115977 – Elucidating the etiology of SPAST-based Hereditary Spastic Paraplegia
- Lisa Dean Moseley Foundation – Using “Mini-Brains” from Patient Derived Pluripotent Stem Cells as the Models to Investigate Novel Microtubule-Based Mechanisms and Therapies for Tauopathies.
- Drexel University funding PA Dept of Health CURE (SAP # 4100083087) – Examine Tau-Based Mechanisms and Therapies for Organophosphate-toxicity Using Human Organoids
Research Highlights
Back to Top