Research
Project 1: Generation and termination of calcium signals in astrocytes
Like neurons, astrocyte processes are decorated with receptors and transporters for numerous neurotransmitters, allowing astrocytes to sense and regulate multiple aspects of brain function. Unlike neurons, astrocytes are not electrically active (i.e., their activation does not lead to the generation of action potentials). Instead, the activation of astrocytic neurotransmitter receptors and transporters is frequently coupled, either directly or indirectly, to increases in [Ca2+]i. There are multiple sources for these Ca2+ increases, including IP3 receptor–mediated Ca2+ release from the endoplasmic reticulum, release from mitochondria, and influx across the plasma through ion channels and exchangers.
Calcium signals in astrocytes are highly varied, ranging from small, local, uncoordinated elevations in [Ca2+] to large, somatic [Ca2+] increases that are coordinated between many cells, such as occur in response to adrenergic signaling in vivo. Increases in intracellular [Ca2+] are implicated in many astrocyte functions.
Processes of an astrocyte in an organotypic hippocampal culture transfected with a membrane-tethered mCherry protein and a genetic calcium indicator (Lck-GCaMP5G). Calcium transients within the astrocyte processes are generally small, discrete events that are rarely coordinated between processes. From Jackson JG et al JNeurosci (2015)
While a great deal of work has concentrated on elucidating mechanisms resulting in increase in [Ca2+]i, the mechanisms regulating the cessation of these signals are not well resolved. An ongoing project in the Jackson Lab is to better understand mechanisms terminating and restricting Ca2+ signals in astrocytes, to determine how altered Ca2+ clearance contributes to changes in astrocyte function during tissue injury or inflammation, and to determine how altered clearance impacts astrocyte function.
Project 2: Regulation and role of astrocytic mitochondria in synaptic development and maintenance
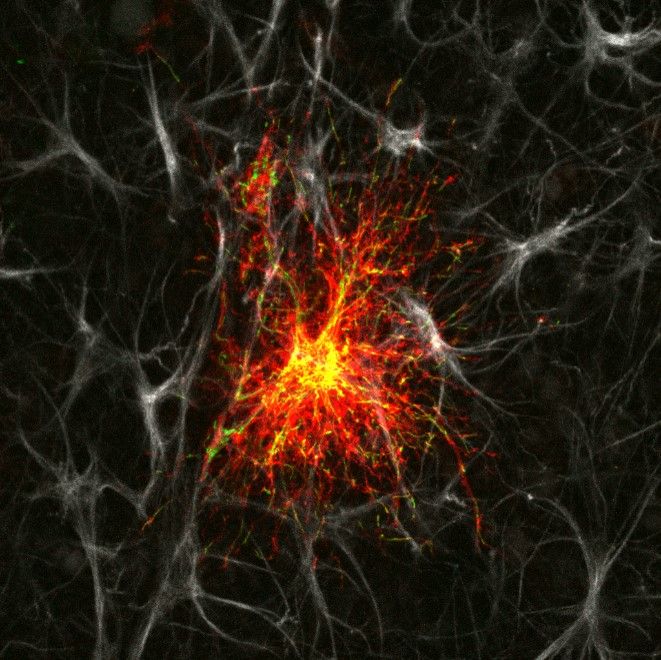 Single astrocyte in an organotypic hippocampal slice culture. Astrocyte has been transfected via gene-gun with a membrane-targeted mCherry fluorescent protein (red) and a mitochondrially-targeted green fluorescent protein. Both constructs were driven by a minimal glial fibrillary acidic protein promoter (GFAABC1D). The slice was fixed and immuno-stained with an anti-GFAP antibody (grey).
Single astrocyte in an organotypic hippocampal slice culture. Astrocyte has been transfected via gene-gun with a membrane-targeted mCherry fluorescent protein (red) and a mitochondrially-targeted green fluorescent protein. Both constructs were driven by a minimal glial fibrillary acidic protein promoter (GFAABC1D). The slice was fixed and immuno-stained with an anti-GFAP antibody (grey).
Astrocyte activity and metabolism is heavily integrated with neuronal activity. While classically, we think of astrocytes as being principally glycolytic, in fact astrocytes possess significant capacity for oxidative (mitochondrial) metabolism. Mitochondria are indeed found throughout the processes of astrocytes and are enriched in the peripheral processes that appose synaptic elements.
The stopping and accumulation of mitochondria at these sites is mediated by neuronal activity–dependent increases in [Ca2+]i within the astrocyte. Astrocytic mitochondria accumulate/buffer Ca2+ signals within these processes and likely influence local metabolism and signaling. One of the projects currently being pursued by the Jackson Lab is to understand the role that astrocytic mitochondria play in the development and maintenance of synaptic structures and signaling.
Our research combines optical imaging with traditional molecular/genetic and electrophysiologic techniques to interrogate the signaling that occurs between astrocytes and neurons. We utilize organotypic “slice” cultures as well as in vivo models to address these questions.
Astrocyte transduced in vivo using an adeno-associated viral vector (AAV2/5) to facilitate expression of a mitochondrially targeted green fluorescent protein (EGFPmito). Mitochondria are visible throughout the astrocyte arborization.