Why Do mRNA Vaccines Cause Strongest Immune Response in Younger Individuals? Lipid Nanoparticles Offer Some Answers.
 By Greg Richter
By Greg Richter

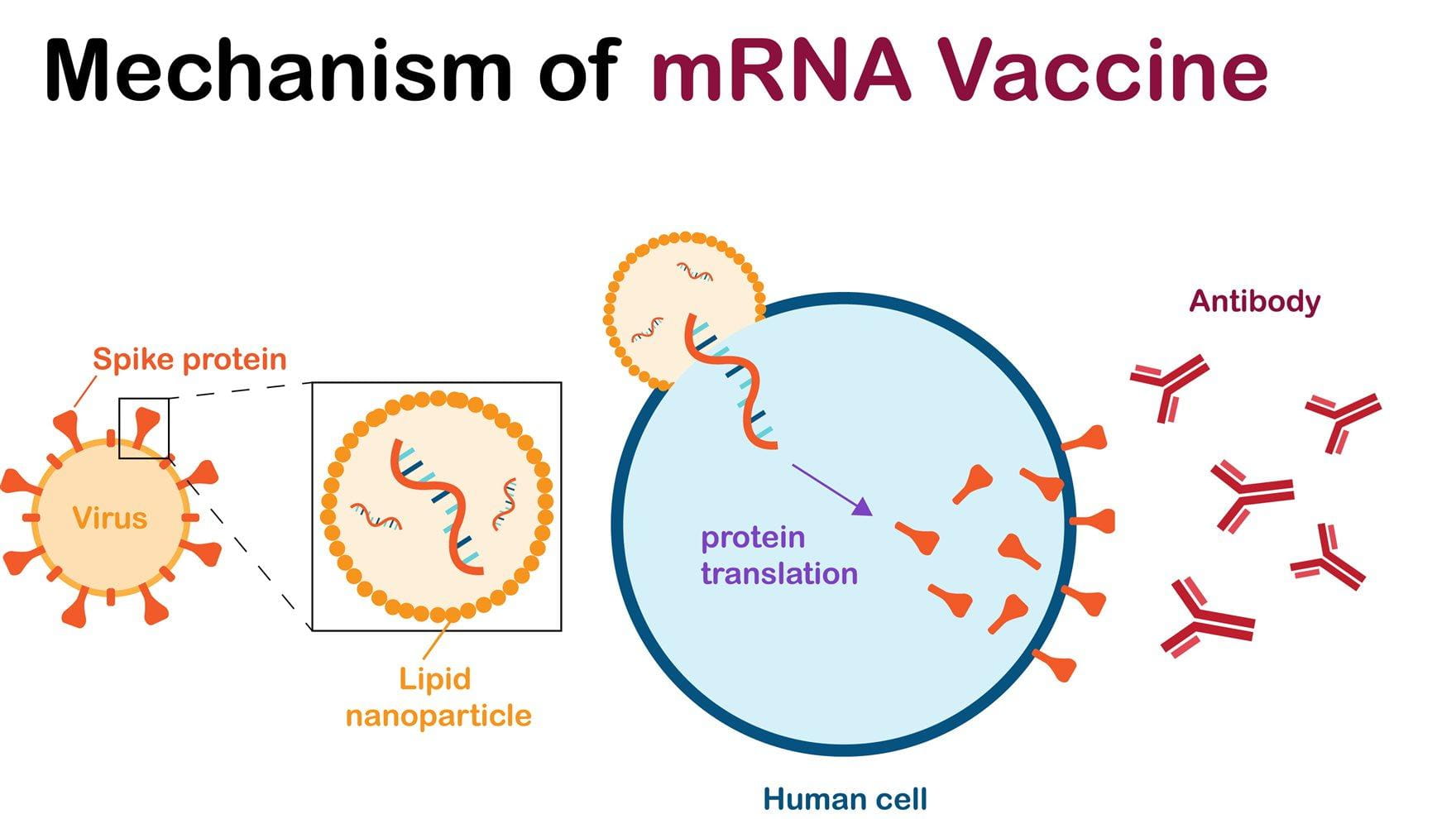
Although the mRNA COVID-19 vaccines from Pfizer-BioNTech and Moderna are safe and effective at preventing severe illness in adults and children, including immunocompromised individuals, researchers have noticed that these shots continue to be the most efficient and effective in younger individuals than in older adults.
Researchers at Drexel University’s College of Medicine and the Perelman School of Medicine at the University of Pennsylvania studied the role of lipid nanoparticles, or LNPs — a vital component of these vaccines that delivers the mRNA cargo to cells, including to cells of the innate immune system (or response cells). According to a recently published article in the journal Communications Biology, these researchers showed that LNPs are able to stimulate innate immune cells from old and young individuals, however, more efficiently in younger individuals.
The mRNA-based COVID-19 vaccines — given the green light from the U.S. Food and Drug Administration and the European Medicine Agency back in 2021 — use messenger RNA that instructs cells to make proteins. The mRNA technology behind those vaccines was developed at Penn Medicine.
In the case of COVID-19 vaccines, the mRNA instruct the cells to make the SARS-CoV-2 spike protein, one component of the virus, to help the immune system recognize it and build up antibodies to fight and neutralize the virus upon subsequent infection.
Although seemingly simple, these vaccines rely on a critical packaging made of lipids (fat-like molecules) to protect, and to deliver, the mRNA into cells. The packaging is composed of lipid nanoparticles. These LNPs are usually made of four types of lipids, including a lipid that becomes positively charged under slight acidic conditions to allow the packaging of the negatively charged mRNA during vaccine preparation. Other components of LNPs include cholesterol — the same found in our bodies and in food — and other molecules that help give structure and keep the particles stable. As soon as LNPs enter cells, they release the mRNA.
Without LNPs, there would be no mRNA vaccine against COVID-19, or infectious diseases, such as respiratory syncytial virus (RSV), cytomegalovirus (CMV) and others.
In their study, the team of researchers looked at the effect of LNPs using human-derived cell cultures. The researchers separated immune cells, called monocytes, from the blood of 18 healthy adult donors aged between 25 and 75 years.
“The adjuvanted effect of mRNA-based vaccine is delivered primarily by ionizable lipids in the LNP,” said senior author Elias El Haddad, PhD, a professor in the College of Medicine. “We found that empty LNPs, those with no mRNA, stimulate dendritic cell maturation – during which cells change from the processing stage to stimulate innate immune system pathways that are less efficient in older adults over 65 years compared to younger adults.”
These new findings confirm previous data from co-corresponding author Mohamad-Gabriel Alameh, PhD, a director of the Engineered mRNA and Targeted Nanomedicine Core at Penn Medicine, that ionizable lipids boost vaccine effectiveness. The authors studied the effect of these LNPs on innate immune cells from humans and showed differences between aged and young adults.
The researchers say that LNPs are an important factor in mRNA-based vaccines that help in the initiation of vaccine response. A less efficient response to LNPs in older adults could explain reduced vaccine response in these individuals and highlight the need to improve these responses in the aged population.
“Lower or less efficient immune responses to vaccines in older individuals does not have anything to do with the vaccine platform,” said Alameh. “It stems from inflammation, metabolic activity and genetics.”
The authors stress that understanding the immune responses to LNPs, and to other adjuvants used in vaccines, in the aged population is critical as it may help offer strategies to improve vaccines in, or even tailor vaccines that are specific for, the aged population.
The latest research follows past work by El Haddad and colleagues that focused on developing substances to improve vaccine immune response in elderly adults for another viral infection, HIV. In 2019, El Haddad and colleagues published a study identifying a substance called adenosine deaminase-1, which they have patented, and the role it plays in T cell function among adults with HIV.
“When a pandemic comes you always try to pivot and understand better how to deploy your experience to develop better vaccines for new diseases,” said El Haddad. “When we saw that the immune response in older individuals was less efficient than that of younger individuals to the vaccine, that’s when we looked at the delivery system – the LNP, which is also the adjuvant part of the vaccine, to understand what effects the LNP’s efficiency.”
El Haddad said the next step in the research is to improve the adjuvant effect of the LNP to improve the efficacy of each dose. The team is currently working on multiple strategies including supplementing the LNP with their own adjuvant to see if it is as efficient in older individuals as it is in younger ones.
“The need for better vaccine platforms will likely only grow in the coming years as new infectious diseases emerge along with new opportunities for mRNA vaccines,” said lead author Jennifer Connors, PhD, who participated in the research while completing a doctoral degree in the Drexel College of Medicine and now works in the pharmaceutical industry. “Decades of research into mRNA vaccines led to the COVID vaccines and decades of research to come will yield incredible vaccine advances to combat disease, including cancer.”
This work was supported by a grant from the National Institutes of Health.
Other than El Haddad, Alameh, and Connors, additional authors on the paper include David Joyner , Gina M. Cusimano, Matthew R. Bell, Jennifer Marcy, Bhavani Taramangalam, and Kenneth M. Kim from Drexel, Nathan J. Mege from Tower Health, Paulo J. C. Lin and Ying K. Tam of Acuitas Therapeutics, Drew Weissman of the Perelman School of Medicine at the University of Pennsylvania, and Michele A. Kutzler of Drexel.
Drexel News is produced by
University Marketing and Communications.