“Sorry, I didn’t get your call; my cell phone battery died,” “I wish I could charge my battery in two minutes,” and “My laptop is only three years old, but the battery only lasts me a couple of hours” are common complaints heard as we rely on technology as an integral part of our existence. Imagine not having to charge your cell phone battery for a week or never having to get a new laptop battery. Faculty from Drexel’s Department of Materials Science and Engineering explore this topic in a recent invited “Perspective” article in Nature Energy.
Batteries with long life that charge nearly instantly would transform how we as a society interact with our technology. Anne Stevens Assistant Professor Ekaterina Pomerantseva and Distinguished University and Charles T. and Ruth M. Bach Chair Professor Yury Gogotsi posit that next-generation batteries created by combining different two-dimensional (2D) materials in heterostructured electrodes can greatly improve battery life and charge storage capabilities, altering the technology landscape.
The term “2D materials” is used to describe materials that are created in a single layer, akin to a sheet of paper that is only one or several atoms thick. These unique materials have begun to show great potential for distinct properties that could be applied to a wide variety of applications.
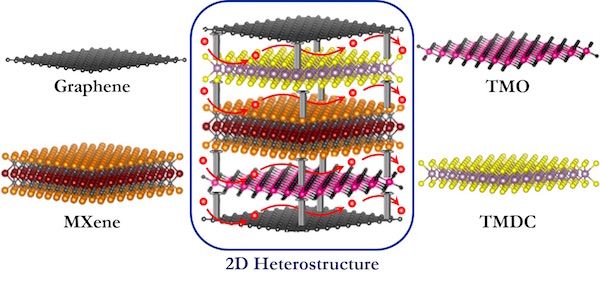 The most commonly known 2D materials are graphene and different graphene modifications, transition metal dichalcogenides (TMDCs), transition metal oxides (TMOs), and transition metal carbides/nitrides (MXenes). Each of these material families exhibits certain advantages for energy storage applications, but at the same time have some disadvantages. “We propose that to eliminate the disadvantages while maintaining all of the advantages of these differing 2D materials is to create what are called 2D heterostructured electrodes composed of alternating layers of different 2D materials that exhibit diverse functionalities,” says Pomerantseva.
The most commonly known 2D materials are graphene and different graphene modifications, transition metal dichalcogenides (TMDCs), transition metal oxides (TMOs), and transition metal carbides/nitrides (MXenes). Each of these material families exhibits certain advantages for energy storage applications, but at the same time have some disadvantages. “We propose that to eliminate the disadvantages while maintaining all of the advantages of these differing 2D materials is to create what are called 2D heterostructured electrodes composed of alternating layers of different 2D materials that exhibit diverse functionalities,” says Pomerantseva.
The single-layer structure of 2D materials is particularly advantageous for energy storage properties, as these thin materials have a high surface area, which allows for the rapid diffusion of ions from an electrolyte, a liquid used to transport ions between two electrodes. The rapid movement of ions on the surface allows for a battery to charge more quickly. In addition, 2D materials make room for a denser packing of ions between the layers, which leads to the battery holding the charge for longer. While there is a chance that the solvent used in the electrolyte has the potential to cause an adverse chemical reaction with the electrode within the battery causing reduced battery performance, the promise of 2D materials for improved battery performance far outweighs the potential challenges.
To construct these 2D heterostructures requires a parallel orientation, or interface, of the layers of different 2D materials (see accompanying figure). “The interface of materials in this way optimizes their unique properties,” says Gogotsi. “For example, by combining alternating layers of TMO and graphene,” continues Gogotsi, “both a high capacity of oxide and high electronic conductivity of carbon can be achieved in the resulting 2D heterostructure, leading to a battery with both high energy and high power.”
It is possible to modify the 2D heterostructures further by incorporating various inorganic ions, organic molecules, or even polymers, known as species, between the layers. The incorporation of these interlayer species can lead to several advancements in electrochemical properties. Interlayer species can expand the interlayer spacing, allowing for more electrochemically cycling ions to be incorporated between the layers, leading to higher capacity and, thus, energy density of the batteries, as cited above. Additionally, because of the interactions between the layers and interlayer species, the structural stability of the electrodes throughout multiple cycles of ions undergoing insertion and extraction can be improved. If the interlayer species are charged, their presence between the layers can affect the diffusion of electrochemically cycled ions, including acceleration of the diffusion, which will lead to batteries with greater power.
Using 2D heterostructured electrodes with appropriately selected interlayer species holds great promise to create a battery that will not only charge more quickly and hold a single charge longer, but will also last longer over many charge and discharge cycles. “If such a battery is used to power one’s car,” shares Pomerantseva, “the car will be capable of a greater driving range before needing to be re-charged, the re-charging process will take a very short period of time, and the owner of the car will probably want to buy a new car well before the light indicating that the battery needs to be replaced is illuminated.”