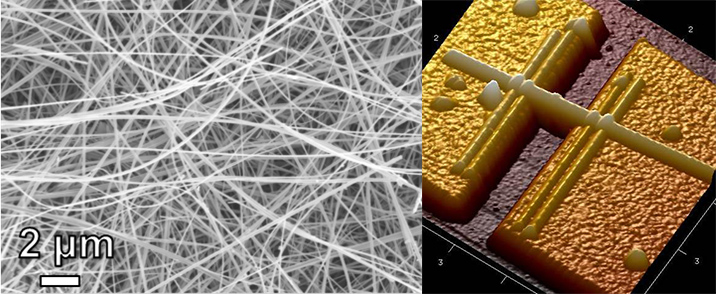
Tunnel structured manganese nanowires used to develop Na-ion batteries with enhanced performance: (left) SEM image of nanowires synthesized using hydrothermal treatment approach, (right) a tapping mode AFM image of a single nanowire placed on a platform enabling measurements of electromechanical properties. Combined bulk and single nanowire measurements are essential to better understand the mechanism of sodium cycling in tunnel manganese oxide nanowires.
Assistant Professor Ekaterina Pomerantseva has received a three-year $224,772 NSF grant award through the Division of Chemical, Bioengineering, Environmental and Transport Systems for her project “High-performance nanowire cathodes with stabilized microporous tunnels for Na-ion batteries,” a collaborative effort with Professor Arunkumar Subramanian from the Department of Mechanical and Industrial Engineering at the University of Illinois in Chicago.
Rechargeable batteries help to enable a sustainable energy future by providing stable storage and demand-matched delivery of electricity generated from fluctuating renewable sources such as wind or sun. Currently, lithium-ion batteries offer the best performance in terms of capacity, power delivery, and longevity, however, lithium is a limited resource with respect to both its total supply in the Earth's crust and its geographic availability. Rechargeable batteries which use sodium ions instead of lithium ions for storing charge mitigate the possibility of lithium scarcity in the future, because sodium is a very abundant element. The drawback of sodium ions is that they are much larger ion than lithium ions, and this large size creates a variety of operational problems within a rechargeable battery, particularly swelling of the battery electrode during charging and discharging. Through a nanotechnology-based approach, Pomerantseva’s project will develop new cathodes for sodium-ion batteries using hollow nanowires which confine the sodium ions within a tunnel structure. The nanowires will be made of magnesium oxide with tunnel diameters of about 2 nanometers. The key innovation is that by confining the sodium ions within the tunnel, the swelling can be controlled and storage capacity can be increased simultaneously. The scientific outcomes of this project can be used to help research with other future metal ion battery systems based on magnesium, aluminum and potassium, where similar issues might occur.
This is Pomerantseva’s second NSF grant to support lithium-ion alternatives in 2016. She previously received a three-year $360,000 award for her project “Advanced Electrochemistry of Na-ion Battery Cathodes Through Chemically Controlled Materials Synthesis.”