A team of Drexel Materials researchers has designed a transition metal oxide that exhibits unique electrical properties, making it a potential ultrafast thermal conductor that could transform the field of nuclear engineering.
Materials science and engineering assistant professor James Rondinelli and his colleague have designed a metallic material that has the potential to serve as a thermal sensor for nuclear reactors. The material has possible applications in advanced electronics and optics as well.
Most metallic materials do not sustain electric dipoles—asymmetrically distributed electric charges in the material—and therefore are not polar. Nevertheless, approximately 30 of these unusual metals are known to exist and are able to sustain such electric dipoles. Of these known compounds, only three contain oxygen and transition metal elements from the periodic table; one of which was recently reported this fall in Nature Materials and experimentally made through high-pressure synthesis methods.
In this research, funded by the Army Research Office Young Investigator Program, and reported in an article in Nature Communications on March 17, 2014, Professor James Rondinelli and his colleague, postdoctoral research fellow Dr. Danilo Puggioni, predict an additional fourth transition metal oxide member of this polar metal family using advanced computational materials science methods based on solving quantum mechanical equations describing electrons in solids.
Such polar metals were hypothesized to have existed in the early 1960s, yet with little theoretical understanding of how to discover them. By examining the crystal structure of the known metals, Rondinelli and Puggioni were able to show that the manner in which the asymmetric charge distribution arises from the geometric arrangement of atoms at the sub-nanometer scale is key to understanding how to discover more compounds.
Together they created a classification system for these metals, a first for these unique materials, based on the way the atoms in the structure destroy inversion symmetry, that is, whether or not an identical atom or set of atoms exists diametrically opposite and at an equal distance from some point in the crystal.
“Understanding the classification of these materials is significant because it challenges the way we think about what it means to be simultaneously a metal [able to conduct electricity] and to have an asymmetric distribution of charges in a solid,” says Rondinelli.
Using the new classification system, Rondinelli and Puggioni were then able to design the fourth conductive and polar oxide material.
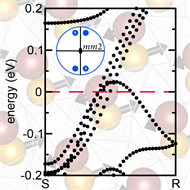
Many of the 30 existing polar metals are superconductors. While Rondinelli’s new material is not predicted to be a superconductor, he and his colleague surmise that it could be, and this material may be an ultrafast heat conductor, owing to the anisotropy in the crystal structure from the alignment of the layer dipoles. Unlike most materials, which have the greatest thermopower—a measure of the voltage induced by a temperature variation across the material—along the least conductive direction, this material has the greatest thermopower along the direction in the crystal that is most conducting, which would provide a faster response, especially where stability under extreme conditions, speed, and the ability to measure heat fluxes of high density are key requirements, for example, in thermal (heat) radiation detectors.
The material, (Sr,Ca)RuO6, is currently in the theoretical stage, but Rondinelli and his colleague are working with experimental groups around the country to realize this compound in the laboratory. The idea is to combine two materials that are known to exist, SrRuO3 and CaRuO3, at the atomic scale in an ordered fashion, the result being an atomic scale superlattice, or composite, that repeats each of these two materials in a periodic fashion with a spacing of approximately 4 nm. These two materials have never been studied in this geometry or made at this length scale together and the Drexel researchers anticipate their new finding will ignite renewed interest in Ru-based oxides.
“The way these materials behave and the reasons for their stability are rather unconventional, yet our classification scheme provides a general design strategy that could guide the discovery and realization of many more polar metals,” says Rondinelli. “I don’t believe these materials are as rare as is currently thought despite their counterintuitive nature; researchers may have simply been looking in the wrong places.”
Designing new materials and the ability to predict new compounds that can better serve society are key initiatives of President Obama’s Materials Genome Initiative (MGI). This recent discovery is one way in which Rondinelli’s Materials Theory and Design Group at Drexel is involved in meeting the challenge on which the MGI is formulated—the discovery of new functional materials in an accelerated fashion. Along these lines, ideas for how to halve the time from materials discovery in the laboratory to commercialization described by Rondinelli and his collaborators, was also recently featured inThe Bulletin of the American Ceramic Society, the focus being that advances in materials understanding and discovery will greatly benefit from unconventional multidisciplinary efforts.